含酪氨酸自组装β-片肽对巨噬细胞极化和炎症反应的影响
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
自组装肽(SAP)是一种完全定义的纳米生物材料,为控制纳米结构和化学属性以研究和操纵细胞信号提供了前所未有的机会。为了研究化学和形态特征对原生免疫中炎症信号转导的影响,我们设计了五种β片状SAP:EFEFKFEFK(EF8)、YEFEFKFEFK(YEF8)、EFEFKFEFKY(EF8Y)、YEFEFKFEFKY(YEF8Y)和EYEFKFEFK(EYF8)(F:苯丙氨酸;E:谷氨酸;K:赖氨酸;Y:酪氨酸)。肽序列中酪氨酸的位置决定了自组装成纳米结构的程度,所有 SAP 都能自组装成细的纳米纤维(d ≈ 3.8 ± 0.4 nm),而 YEF8 和 EF8 序列则显示出关联捆绑的倾向。这些不同的 SAP 可诱导单核细胞模型 THP-1 细胞衍生巨噬细胞(MΦs)产生截然不同的炎症反应。可溶性 EF8 纳米纤维(2 mM)可诱导抗炎反应并向 M2 状态极化,而 YEF8(2 mM)则倾向于诱导促炎反应并向 M1 状态极化。在我们的模型中,EF8Y、YEF8Y 和 EYF8 SAPs 没有诱导炎症反应。这些结果通过使用人类捐献者的外周血单核细胞(PBMCs)衍生的 MΦs 得到了验证,证实了 EF8 和 YEF8 SAPs 分别作为组织修复的可能协调者或促炎状态的诱导者的关键作用。在 20 mM 水凝胶上培养的 THP-1 衍生 MΦs 也获得了相同的 MΦs 极化反应。这些研究结果将有助于利用该系列 SAP 作为免疫调节纳米生物材料,从而有可能改变各种疾病进展过程中的炎症过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
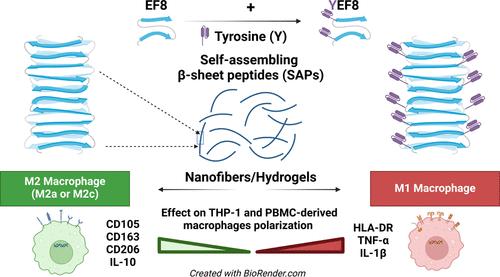
Effect of Tyrosine-Containing Self-Assembling β-Sheet Peptides on Macrophage Polarization and Inflammatory Response
Self-assembling peptides (SAPs) are fully defined nanobiomaterials offering unprecedented opportunities to control nanostructure and chemical attributes to investigate and manipulate cellular signals. To investigate the influence of chemical and morphological characteristics on inflammatory signaling in native immunity, we designed five β-sheet SAPs: EFEFKFEFK (EF8), YEFEFKFEFK (YEF8), EFEFKFEFKY (EF8Y), YEFEFKFEFKY (YEF8Y), and EYEFKFEFK (EYF8) (F: phenylalanine; E: glutamic acid; K: lysine, Y: tyrosine). The position of tyrosine in the peptide sequence dictated the self-assembly into nanostructures, with all SAPs self-assembling into thin constituent nanofibers with d ≈ 3.8 ± 0.4 nm, and sequences YEF8 and EF8 showing a propensity for associative bundling. These distinct SAPs induced contrasting inflammatory responses of monocytic model THP-1 cells-derived macrophages (MΦs). Presence of soluble EF8 nanofibers (at 2 mM) induced an anti-inflammatory response and polarization toward an M2 state, whereas YEF8 (at 2 mM) displayed a tendency for inducing a pro-inflammatory response and polarization toward an M1 state. EF8Y, YEF8Y, and EYF8 SAPs did not induce an inflammatory response in our models. These results were validated using peripheral blood mononuclear cells (PBMCs)-derived MΦs from human donors, confirming the critical role of EF8 and YEF8 SAPs as possible orchestrators of the repair of tissues or inducers of pro-inflammatory state, respectively. The same MΦs polarization responses from THP-1-derived MΦs cultured on 20 mM hydrogels were obtained. These findings will facilitate the utilization of this family of SAPs as immunomodulatory nanobiomaterials potentially changing the course of inflammation during the progression of various diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


