通过光诱导n-肉桂醛腙级联磺化环化制备c5 -磺酰基化四氢吡啶
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以N′-苄基-N-肉桂酰乙肼为新骨架,通过光催化碳磺酰化/环化工艺,在四氢吡啶的c5位上直接安装了磺酰基官能团。用芳基或烷基磺酰氯作为磺酰自由基源,得到了各种取代的c5磺酰化四氢吡啶,收率很高。这个反应是通过在肉桂基的C2位置选择性地添加磺酰自由基,然后在腙CH = N键上形成6-内三角环来实现的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
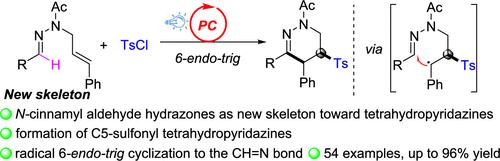
Access to C5-Sulfonylated Tetrahydropyridazines via Photoinduced Cascade Sulfonylation-Cyclization of N-Cinnamyl Aldehyde Hydrazones
Direct installation of a sulfonyl functional group into the C5-position of tetrahydropyridazine was achieved using N′-benzylidene-N-cinnamylacetohydrazide as a new building skeleton via the photocatalytic carbosulfonylation/annulation procedure. Various substituted C5-sulfonylated tetrahydropyridazines were obtained in good to excellent yields employing aryl- or alkylsulfonyl chlorides as the sulfonyl radical sources. This reaction was realized through the selective addition of sulfonyl radical to the C2 position of cinnamyl followed by the 6-endo-trig annulation to the hydrazone CH═N bond.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


