硝基烃森田-贝利斯-希尔曼加合物与磺酰基邻苯酞Hauser-Kraus环法制备菲萘醌融合苯并西平
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
硝基烯的森田-贝利斯-希尔曼(MBH)加合物与磺酰基邻苯酞的haser - kraus环化提供了获得菲和萘醌融合二氢苯并西平的途径。H-K环化使萘醌以受保护形式携带一个关键醇基团,与甲醛反应生成萘醌融合四环氧平。这些醇通过氧化和与β-酮酯的[3 + 3]环化产生功能化的菲。本文章由计算机程序翻译,如有差异,请以英文原文为准。
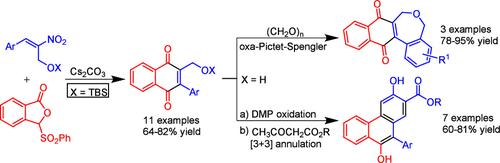
Synthesis of Phenanthrenes and Naphthoquinone Fused Benzoxepines via Hauser–Kraus Annulation of Sulfonylphthalide with Morita–Baylis–Hillman Adducts of Nitroalkenes
The Hauser–Kraus annulation of sulfonylphthalide with Morita–Baylis–Hillman (MBH) adducts of nitroalkenes provides access to phenanthrenes and naphthoquinone fused dihydrobenzoxepines. The H–K annulation results in naphthoquinones bearing a key alcohol group in protected form, which leads to naphthoquinone fused tetracyclic oxepines upon reaction with formaldehyde. These alcohols yield functionalized phenanthrenes via oxidation and [3 + 3] annulation with β-ketoesters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


