一种基于供体-受体共价三嗪框架的“开-关-开”电化学发光配体传感器在缺口内切酶驱动的DNA行走机辅助下检测黄曲霉毒素B1
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
构建了一种基于供体-受体(D-A)共轭共价三嗪框架(CTF)的“开关”信号可切换电化学发光(ECL)适体传感器,在核酸切酶(Nb.BbvCI)驱动的DNA行走机的辅助下,对黄曲霉毒素B1 (AFB1)进行灵敏、准确的检测。D-A共轭CTF由2,4,6-三(4-甲苯基)-1,3,5-三嗪(TFPT)和三(4-氨基苯基)甲烷(TAPM)(标记为TFPT-TAPM-CTF)反应生成,同时作为优良的ECL发射器和锚定生物探针的平台。tfpt - ttap - ctf的高ECL响应可以通过锚定的cy5标记单链DNA (Cy5-ssDNA)通过ECL共振能量转移来抑制。此外,在afb1靶向适体和DNA行体(Hp)之间产生的双链DNA的固定化降低了tfpt - ttap - ctf的ECL反应。当检测AFB1时,适体从双链DNA中分离以捕获特定靶标,导致自由Hp链与Cy5-ssDNA杂交。在Nb的帮助下。BbvCI释放的部分Cy5-ssDNA在一定程度上有助于恢复tfpt - ttap - ctf的ECL应答,进一步解放Hp链,使其自主结合到另一个Cy5-ssDNA上,触发新的切割过程。在1.0 pg·mL-1 ~ 5.0 × 104 pg·mL-1范围内,ECL感应传感器的检测限为0.59 pg·mL-1,具有较高的选择性和良好的实用性。本工作拓宽了CTF在食品安全领域的应用,为食品中真菌毒素的灵敏、精确检测提供了一种新的检测策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
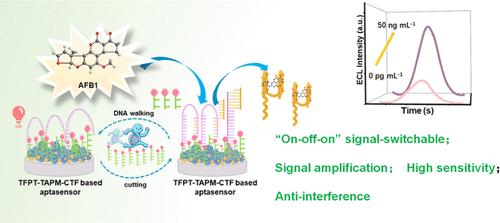
A Donor–Acceptor Covalent Triazine Framework-based “On–Off–On” Electrochemiluminescence Aptasensor for Detecting Aflatoxin B1 with the Assistance of Nicking Endonuclease-powered DNA Walking Machine
A designed “on–off–on” signal-switchable electrochemiluminescence (ECL) aptasensor based on a donor–acceptor (D–A) conjugated covalent triazine framework (CTF) has been constructed for the sensitive and accurate detection of aflatoxin B1 (AFB1) with the assistance of a nicking endonuclease (Nb.BbvCI)-powered DNA walking machine. The D–A conjugated CTF, generated from the reaction between 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (TFPT) and tris(4-aminophenyl)methane (TAPM) (denoted as TFPT-TAPM-CTF), simultaneously serves as a superior ECL emitter and a platform for anchoring the bioprobe. The high ECL response of TFPT-TAPM-CTF can be quenched by the anchored Cy5-labeled single-strand DNA (Cy5-ssDNA) via ECL resonance energy transfer. Furthermore, the immobilization of the double-strand DNA generated between the AFB1-targeting aptamer and the DNA walker (Hp) reduces the ECL response of TFPT-TAPM-CTF. When detecting AFB1, the aptamer separates from the double-strand DNA to capture specific targets, resulting in the hybridization of the free Hp strand and Cy5-ssDNA. With the assistance of Nb.BbvCI, the released partial Cy5-ssDNA helps recover the ECL response of TFPT-TAPM-CTF to some extent, further liberating the Hp strand to autonomously bind to another Cy5-ssDNA and trigger a new cleavage process. In addition to its high selectivity and promising practicality, the constructed ECL aptasensor shows an ultralow detection limit of 0.59 pg·mL–1 within a wide range from 1.0 pg·mL–1 to 5.0 × 104 pg·mL–1. This work broadens the application of CTF in the food safety field and provides a new aptasensing strategy for the sensitive and precise inspection of mycotoxins in food products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


