蛋白质顺磁核磁共振光谱中优异的铁(II)结合标签
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
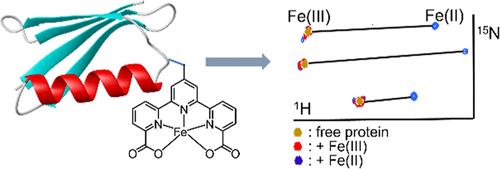
大多数第一系列过渡金属离子都有一个或多个不成对的电子,并在顺磁性方面表现出很大的变化。一些过渡金属离子,包括Co(II)和镧系离子[Ln(III)]的磁各向异性,已经在蛋白质中得到了很好的NMR研究。相比之下,除了含有血红素基序或铁硫簇的铁(II)配合物外,很少有例子报道了蛋白质的磁各向异性。在这里,我们证明了[2,2 ':6 ',2″-三吡啶]-6,6″-二羧酸(TDA)是一种很好的铁结合配体。它在水溶液中形成稳定的铁络合物,并表现出明显的铁结合特性。TDA与Fe(II)和Fe(III)均形成1:1稳定的配合物,但Fe(II)在配合物中呈现高自旋态。TDA片段可以位点特异性地附着在蛋白质上,其蛋白质偶联物在与Fe(II)的配合物中产生相当大的假接触位移(PCSs),这比常用的金属结合标签要大。相反,蛋白质- tda - Fe(III)复合物在蛋白质信号中产生可忽略的顺磁弛豫增强(PRE)效应,表明蛋白质- tda复合物中的Fe(III)处于低自旋状态。蛋白质- tda - fe (II)复合物的高稳定性使人们能够在细胞裂解物中精确测量PCSs,即使存在其他过渡金属离子和过量的谷胱甘肽。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Excellent Fe(II) Binding Tag in Protein Paramagnetic NMR Spectroscopy
Most first series of transition metal ions have one or more unpaired electrons and show great variations in the paramagnetic property. The magnetic anisotropy of some transition metal ions, including Co(II), as well as lanthanide ions [Ln(III)], has been well examined in proteins by NMR. In contrast, few examples of Fe(II) complexes reporting the magnetic anisotropy were analyzed in proteins, except for the ones containing a heme motif or iron–sulfur clusters. Here, we showed that [2,2′:6′,2″-terpyridine]-6,6″-dicarboxylic acid (TDA) is an excellent iron-binding ligand. It forms a stable iron complex in aqueous solution and demonstrates distinct iron-binding properties. TDA forms a 1:1 stable complex with both Fe(II) and Fe(III), but Fe(II) presents a high-spin state in the complex. The TDA moiety can be site-specifically attached to a protein, and its protein conjugate generates sizable pseudocontact shifts (PCSs) in complex with Fe(II), which are larger than those of commonly used metal binding tags. In contrast, the protein–TDA–Fe(III) complex produces negligible paramagnetic relaxation enhancement (PRE) effects in the protein signals, indicating a low-spin state of Fe(III) in the protein-TDA complex. The high stability of the protein-TDA-Fe(II) complex allows one to measure accurate PCSs in cell lysate even in the presence of other transition metal ions and an excess of GSH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


