采用不同锂盐包覆LNMO阴极稳定电极结构和提高电池性能的牺牲预锂化
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
无钴高压尖晶石型阴极LiNi0.5Mn1.5O4 (LNMO)具有高能量和功率密度,是下一代锂离子电池(LIBs)的理想候选材料。但其4.7 V的高工作电压会导致电解液分解,造成结构损坏。此外,在早期循环中活性锂的不可逆损失降低了容量性能,阻碍了其商业应用。为了充分利用LNMO的催化作用和牺牲盐的预锂化,本工作将牺牲盐(Li2C2O4、Li2CO3和CH3COOLi)与LNMO混合,形成预锂化电极(LNMO- sac),并研究不同牺牲盐对锂离子电池性能的影响。结果表明,LNMO能有效催化牺牲盐的分解,促进循环后电极-电解质界面的形成,以减轻电解质分解对电极结构的破坏。与其他牺牲盐相比,Li2C2O4高效补充了活性锂,提高了电池的电化学性能。因此,LNMO- li2c2o4在1C下的放电容量为137.9 mAh g-1,循环500次后的容量保持率为85.9%,优于未预锂化的LNMO。此外,LNMO-LO/Gr充满电池的初始容量为117.9 mAh g-1,在300次循环后保持79.4 mAh g-1。这项工作证明了牺牲盐作为锂离子补给策略在实际应用中的可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
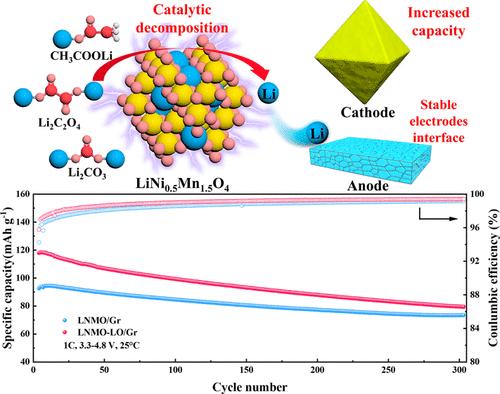
Sacrificial Prelithiation Using Different Lithium Salt-Coated LNMO Cathodes for Stabilizing the Electrode Structure and Enhancing Battery Performance
The cobalt-free, high-voltage spinel-type cathode LiNi0.5Mn1.5O4 (LNMO) exhibits high energy and power densities, rendering it a promising candidate for incorporation into next-generation lithium-ion batteries (LIBs). However, its high operating voltage of 4.7 V can lead to electrolyte decomposition, causing structural damage. In addition, the irreversible loss of active lithium in the early cycles reduces capacity performance, hindering its commercial application. To take full advantage of the catalytic effect of LNMO and the prelithiation of sacrificial salt, this work involved blending sacrificial salts (Li2C2O4, Li2CO3, and CH3COOLi) with LNMO to form prelithiated electrodes (LNMO-Sac) and investigating the influence of different sacrificial salts on the performance of LIBs. The results demonstrate that LNMO could efficiently catalyze the decomposition of sacrificial salts and promote the formation of a more stable electrode–electrolyte interface after cycling to mitigate structure destruction of electrodes caused by electrolyte decomposition. Compared with other sacrificial salts, Li2C2O4 provided a highly efficient supplement of active lithium and improved the electrochemical performance of the battery. Thus, LNMO-Li2C2O4 achieved an excellent discharge capacity of 137.9 mAh g–1 at 1C, and capacity retention of 85.9% after 500 cycles, superior to that of LNMO without prelithiation. Moreover, the LNMO-LO/Gr full cell presented an initial capacity of 117.9 mAh g–1 and retained 79.4 mAh g–1 after 300 cycles. This work demonstrates the feasibility of a sacrificial salt as a lithium replenishment strategy for LNMO in practical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


