n -乙烯酰亚胺与烷基、硅基和酰基自由基的模块化还原自由基-极性交叉酰基迁移反应
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们成功开发了基于 SET 还原法的新型 N → C酰基迁移方案,用于制备功能化 α-氨基酮。除烷基和硅基自由基外,来自二氢喹唑啉酮或酰基肟醋酸酯的酰基也能与烯酰胺发生反应,生成各种 1,4-二酮。这些光催化自由基加成/酰基迁移级联反应具有广泛的底物范围、良好的官能团兼容性和温和的反应条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
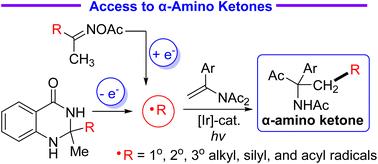
Modular reductive radical-polar crossover-based acyl migration reactions of N-vinylimides with alkyl, silyl, and acyl radicals†
Herein, novel SET reduction-based N → C acyl migration protocols for the preparation of functionalized α-amino ketones were successfully developed. In addition to alkyl and silyl radicals, acyl radicals derived from dihydroquinazolinones or acyl oxime acetates could react with enamides to give various 1,4-diketones. These photocatalytic radical addition/acyl migration cascade reactions feature broad substrate scope, good functional group compatibility, and mild reaction conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


