通过集成膜蒸馏-选择性电渗析从地热卤水中进行可持续的锂回收
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
锂在能源储存系统中的关键作用以及向可再生能源的过渡推动了全球对锂的需求不断上升,因此需要从地热盐水、盐湖和回收电池等创新资源中提取可持续的锂。地热盐水作为清洁能源和锂的来源具有双重优势,与海水(0.18 mg L - 1)相比,其锂浓度(0.01-0.48 g L - 1)更高,与盐湖(0.04-3 g L - 1)相当。此外,与海水(~ 7588)相比,它的Mg/Li比(~ 35.33)要低得多,但仍高于盐湖(~ 6.4)。尽管有这些优势,但由于锂浓度低且存在竞争离子,从地热盐水中回收锂具有挑战性。在此,我们介绍了一种结合膜蒸馏(MD)和选择性电渗析(S-ED)的新方法,用于从地热盐水中高效提取锂和回收水。重点是优化这两个阶段的性能:MD工艺的优化是为了减少盐水体积和比热能耗,而ED工艺的重点是在最佳浓度下最小化电压和比能耗。该混合系统显示了高效锂回收的巨大潜力。在MD阶段,地热水浓缩在15.1、28.1和43.11的不同体积缩小系数(vrf)下,导致后续ED阶段的饲料浓度分别为1 M、2 M和3 M。在自然温度为40°C的地热水中运行MD工艺,使其能量中性,导致比热能节约高达1980.5 kW h m−3。在S-ED阶段,较高的进料浓度提高了锂的选择性,但增加了比能量消耗。在进料浓度为2 M(对应的MD-VRF为28.1)和施加电压为2 V时,性能最佳,每克Li+的比能耗为0.04 kW h,电流效率为4.1%。总体而言,集成的MD-S-ED方法显示了节能锂回收的强大潜力,为创新研究铺平了道路,并为支持循环蓝色经济和绿色过程强化原则的可持续锂提取方法提供了关键见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
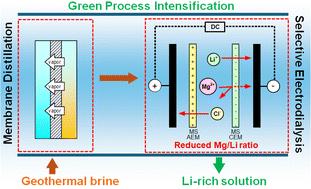
Sustainable lithium recovery from geothermal brine via integrated membrane distillation – selective electrodialysis
The escalating global demand for lithium, driven by its crucial role in energy storage systems and the transition to renewable energy, necessitates sustainable extraction methods from innovative sources such as geothermal brines, salt lakes and recycled batteries. Geothermal brine offers a dual advantage as a source of clean energy and lithium, with higher lithium concentrations (0.01–0.48 g L−1) compared to seawater (0.18 mg L−1) and levels comparable to salt lakes (0.04–3 g L−1). Additionally, it features a much lower Mg/Li ratio (∼35.33) compared to seawater (∼7588), though still higher than that of salt lakes (∼6.4). Despite these advantages, lithium recovery from geothermal brines is challenging due to low lithium concentrations and the presence of competing ions. Herein, we introduce a novel approach that integrates Membrane Distillation (MD) and Selective Electrodialysis (S-ED) for efficient lithium extraction and water recovery from geothermal brines. The focus is on optimizing performance at both stages: The MD process was optimized to reduce brine volume and specific thermal energy consumption, while the ED process was focused on minimizing voltage and specific energy consumption at the optimal concentration. The hybrid system demonstrates strong potential for energy-efficient lithium recovery. At MD stage, geothermal water was concentrated at different volume reduction factors (VRFs) of 15.1, 28.1 and 43.11, resulting in feed concentrations of 1 M, 2 M, and 3 M, respectively, for the subsequent ED stage. Operating the MD process with geothermal water at its natural temperature of 40 °C rendered it energy-neutral, leading to specific thermal energy savings of up to 1980.5 kW h m−3. At S-ED stage, higher feed concentrations improved lithium selectivity but increased specific energy consumption. The optimal performance was observed at a feed concentration of 2 M (corresponding MD-VRF of 28.1) and an applied voltage of 2 V, achieving a specific energy consumption of 0.04 kW h per gram of Li+ and a current efficiency of 4.1%. Overall, the integrated MD–S-ED approach demonstrates strong potential for energy-efficient lithium recovery, paving the way for innovative research and offering key insights for sustainable lithium extraction methods that support the principle of Circular Blue Economy and Green Process Intensification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


