通过掺杂 Ce 调整氧化镁的电子结构和酸碱特性,促进生物质甲酸的室温生产†。
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
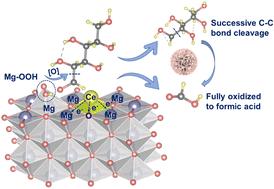
生物质单糖氧化生产甲酸具有重要意义,因为它有可能为传统的化石燃料衍生的甲酸合成方法提供可持续的生物基替代方案。在这项研究中,我们开发了一种Ce - mgo催化剂,通过加入Ce来促进葡萄糖氧化成甲酸。与未改性的MgO相比,Ce-MgO催化剂的碱性位点数量增加,Mg和O位点的电荷密度更高。这些修饰有助于过氧化氢选择性解离形成˙OOH,并增强˙OOH在MgO位点的吸附。这些Mg(OH)(OOH)活性位点的富电子性质通过更有效的电子转移降低了C-C裂解和氧化反应的能垒。因此,该反应可在室温下进行,葡萄糖转化率为97.34%,甲酸收率为93.65%,是所有葡萄糖氧化制甲酸催化剂中性能最高的。此外,Ce-MgO催化剂在催化玉米芯混合糖溶液氧化方面表现出了良好的效果,在30°C下甲酸产率达到49.13%。此外,通过该工艺产生的甲酸可以在室温下就地制氢,突出了从生物质中产生绿色氢的有效和可持续的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tailoring the electronic structure and acid–base properties of MgO by Ce doping promotes biomass-derived formic acid production at room temperature†
Biomass-based monosaccharide oxidation for formic acid production is significant due to its potential to provide a sustainable, bio-based alternative to traditional fossil fuel-derived methods of formic acid synthesis. In this study, we developed a Ce–MgO catalyst by incorporating Ce to enhance the oxidation of glucose to formic acid. Compared to unmodified MgO, the Ce–MgO catalyst exhibits an increased number of basic sites and higher charge densities at the Mg and O sites. These modifications facilitate the selective dissociation of hydrogen peroxide to form ˙OOH species and enhance the adsorption of ˙OOH at the MgO sites. The electron-rich nature of these Mg(OH)(OOH) active sites lowers the energy barrier for the C–C cleavage and oxidation reaction through more efficient electron transfer. Consequently, the reaction can be conducted at room temperature, achieving a 97.34% conversion of glucose and 93.65% yield of formic acid, which represents the highest performance among all glucose oxidation catalysts for formic acid production. Furthermore, the Ce–MgO catalyst demonstrated its efficacy in catalyzing the oxidation of a mixed sugar solution derived from corncob, achieving a formic acid yield of 49.13% at 30 °C. Additionally, the formic acid produced via this process enables in situ hydrogen production at room temperature, highlighting an effective and sustainable approach for generating green hydrogen from biomass.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


