钯催化多氟苯甲酸锌与氟磺酸芳基脱羧交偶联合成多氟双芳基
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了钯催化的多氟苯甲酸锌与氟磺酸芳基脱羧交偶联反应,该反应通过C-O键裂解得到相应的多氟双芳基,产率中高。该反应具有良好的底物范围和广泛的官能团相容性,且易于规模化。在一个烧瓶中,在氟硫酸芳基和钯催化剂共存的情况下,将多氟苯甲酸和Zn(OH)2直接混合,进一步证明了该反应的合成简单性和实用性。进一步的研究表明,氟代硫酸芳基作为芳基化试剂比其他芳基卤化物和假卤化物更强,而多氟苯甲酸锌是一种比镁和钾的同类产品更有效的脱羧多氟芳基化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
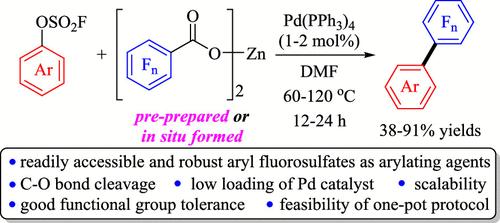
Synthesis of Polyfluorinated Biaryls via Palladium-Catalyzed Decarboxylative Cross-Coupling of Zinc Polyfluorobenzoates with Aryl Fluorosulfates
A palladium-catalyzed decarboxylative cross-coupling of zinc polyfluorobenzoates with aryl fluorosulfates, which proceeded efficiently via C–O bond cleavage to afford the corresponding polyfluorinated biaryls in moderate-to-good yields, was developed. The reactions exhibited both good substrate scope and broad functional group compatibility, and it could be scaled-up easily. The synthetic simplicity and practicability of the reaction was further demonstrated by one-pot manipulation by directly mixing polyfluorobenzoic acid and Zn(OH)2 in the coexistence of aryl fluorosulfate and a palladium catalyst in one flask. Further studies showed that aryl fluorosulfate is more robust than other aryl halides and pseudohalides as a arylating reagent, and zinc polyfluorobenzoate is a more effective decarboxylative polyfluoroarylating agent than their magnesium and potassium counterparts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


