V微合金钢钢筋在模拟碳化混凝土环境中的钝化行为
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用电化学测试(CV、PDP、M-S、EIS、I-T)和表面表征技术(SEM、AFM、TEM、XPS)研究了V微合金钢钢筋在模拟碳化混凝土环境中浸泡10 d的钝化行为。结果表明:实验用钢筋在不同碳化程度的SCP溶液中均能形成具有n型半导体性能的钝化膜;随着碳化的进行,碳钢钢筋的钝化反应减慢,钝化膜因碳化而受损。这使得碳钢钢筋的钝化膜厚度降低到1.6 nm, Ecorr降低到−0.257 V。相反,在碳化作用的影响下,微合金化钢筋中的钒参与钝化膜的形成,并以氧化物的形式逐渐积聚在钝化膜的内层。这些V氧化物的形成抑制了Fe的溶解,提高了钝化反应速率,导致钝化膜的腐蚀电位增加到−0.116 V,膜厚达到3.1 nm。此外,V氧化物可以与氧空位结合,从而使钢筋的钝化膜更加致密。本文章由计算机程序翻译,如有差异,请以英文原文为准。

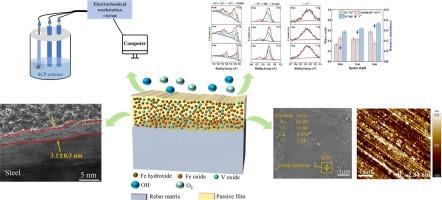
Passivation behavior of V microalloyed steel rebar in simulated carbonated concrete environment
Electrochemical tests (CV, PDP, M-S, EIS, I-T) and surface characterization techniques (SEM, AFM, TEM, XPS) were used to investigate the passivation behavior of V microalloyed steel rebar soaked for 10 d in simulated carbonated concrete environment. The results show that the experimental steel rebars can all form passive film with n-type semiconductor performance in SCP solutions with different carbonation degrees. With the progression of carbonation, the passivation reaction of carbon steel rebar slows down, and the passive film is compromised due to carbonation. This leads to a reduction in the thickness of the passivation film on carbon steel rebar to 1.6 nm and a decrease in Ecorr to −0.257 V. Conversely, under the influence of carbonation, vanadium in microalloyed steel rebar participates in the formation of passive film and gradually accumulates in its inner layer in the form of oxides. The formation of these V oxides inhibits Fe dissolution and enhances the passivation reaction rate, leading to an increase in the corrosion potential of the passive film to −0.116 V and a film thickness of up to 3.1 nm. Furthermore, V oxides can bind to oxygen vacancies, thereby rendering the passive film of steel rebar more compact.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


