两种结构复杂的咪唑衍生物形成双吸附层的协同机制和协同效应
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究了两种配合咪唑衍生物(JMZ和WJN)在1.0 M HCl中对Q235钢的缓蚀协同作用及其机理。电化学结果表明,混合缓蚀剂的缓蚀效率优于单一缓蚀剂,在JMZ与WJN比为4:4时达到98.74 %。扫描电镜(SEM)、原子力显微镜(AFM)和接触角测量表明,碳钢表面的腐蚀程度与缓蚀剂的浓度有关。x射线光电子能谱(XPS)和能谱(EDS)证实了JMZ和WJN在Q235表面的有效吸附。分子动力学模拟表明,协同效应来自于双吸附层,其中JMZ优先吸附在距离Fe(110)表面1.3 Å处,而WJN填补JMZ的空隙并继续吸附在4.57 Å处。当比例为4:4时,吸附能达到−4420.33 kcal·mol−1,使得Cl-和h30 +在溶液中的扩散速率最小。这些发现为开发有效的缓蚀剂提供了双吸附层协同作用的宝贵见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

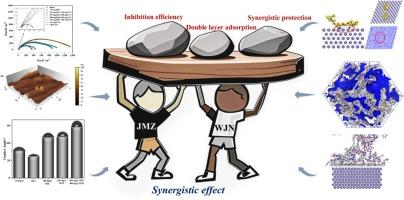
Synergistic mechanism and synergistic effect of two structurally complex imidazole derivatives forming a double adsorption layer
The synergistic effect and mechanism of two complex imidazole derivatives (JMZ and WJN) on corrosion inhibition of Q235 steel in 1.0 M HCl were studied. The electrochemical results demonstrated that the corrosion inhibition efficiency of the mixed inhibitor was superior to that of a single inhibitor, reaching 98.74 % at a JMZ to WJN ratio of 4:4. Scanning electron microscopy (SEM), atomic force microscopy (AFM), and contact angle measurements revealed that the degree of corrosion on the carbon steel surface correlated with the concentration of the inhibitors. X-ray photoelectron spectroscopy (XPS) and energy dispersive spectroscopy (EDS) confirmed the effective adsorption of both JMZ and WJN on the Q235 surface. Molecular dynamics simulations suggest that the synergistic effect arises from a double adsorption layer, where JMZ preferentially adsorbs at a distance of 1.3 Å from the Fe (110) surface, while WJN fills the gaps in JMZ and continues to adsorb at 4.57 Å. At the 4:4 ratio, the adsorption energy reaches −4420.33 kcal·mol−1, minimizing the diffusion rates of Cl- and H3O+ in the solution. These findings provide valuable insights into the synergistic effect of dual adsorption layers in developing effective corrosion inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


