异金属锰(III)与希夫碱(H2SALPN)和双氰金属酸盐配位聚合物
IF 1.1
3区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
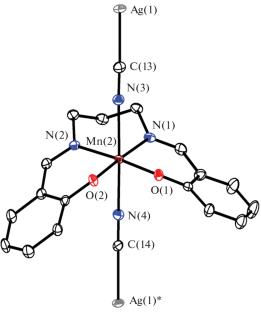
首次获得了四齿(N2O2)席夫碱(L)和双氰金属酸盐[Mn(L)M(CN)2]n配合物的单晶,其中L = Salpn2 - = n, n '-双(水杨基)-1,3-二氨基丙烷,M = Ag+ (I), Au+ (II)。通过x射线衍射(CCDC no. 1)测定了I和II的分子结构。2351118 (i), 2351119 (ii))。结果表明,在这些化合物的晶体结构中,双氰金属酸阴离子[M(CN)2]是将Mn(III)基团与席夫碱结合成一维结构的桥梁。利用量子化学计算,在C-H…Ag/Au接触点附近发现了(3,-1)型临界点;这证明了这些原子之间存在弱氢键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Heterometallic Manganese(III) Coordination Polymers with Schiff Bases (H2SALPN) and Dicyanometallates
Single crystals of the Mn(III) complexes with tetradentate (N2O2) Schiff bases (L) and dicyanometallates [Mn(L)M(CN)2]n, where L = Salpn2– = N,N'-bis(salicylidene)-1,3-diaminopropane, M = Ag+ (I), Au+ (II), were obtained for the first time. The molecular structures of I and II were determined by X-ray diffraction (CCDC no. 2351118 (I), 2351119 (II)). It was found that the dicyanometallate anion [M(CN)2]ˉ in the crystal structure of these compounds acts as a bridge binding the Mn(III) moieties with the Schiff base into 1D. Using quantum chemical calculations, the (3, –1) type critical points were found near the C‒H…Ag/Au contact; this attests to the existence of weak hydrogen bonds between these atoms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Coordination Chemistry
化学-无机化学与核化学
CiteScore
2.40
自引率
15.80%
发文量
85
审稿时长
7.2 months
期刊介绍:
Russian Journal of Coordination Chemistry is a journal that publishes reviews, original papers, and short communications on all aspects of theoretical and experimental coordination chemistry. Modern coordination chemistry is an interdisciplinary science that makes a bridge between inorganic, organic, physical, analytical, and biological chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


