硝酸铀酰与某些酰胺配体的配位化合物
IF 1.1
3区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
通过 UO2(NO3)2 与酰胺 L(L = 乙酰胺、N,N-二甲基乙酰胺、丙酰胺、戊酰胺、苯甲酰胺、N-甲基脲)在水中的反应,制备了六种配位化合物 [UO2(L)2(NO3)2]。通过元素分析、红外光谱以及粉末和单晶 X 射线衍射,确定了产物的组成和结构。量子化学计算证实了所获化合物的分子结构和吸收带的分配。本文章由计算机程序翻译,如有差异,请以英文原文为准。
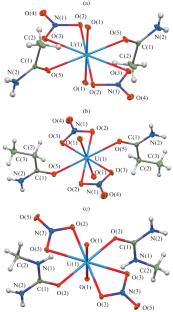
Coordination Compounds of Uranyl Nitrate with Some Amide Ligands
Six coordination compounds [UO2(L)2(NO3)2] were prepared by the reaction of UO2(NO3)2 with amide L (L = acetamide, N,N-dimethylacetamide, propanamide, valeramide, benzamide, N-methylurea) in water. The composition and the structure of the products were established by elemental analysis, IR spectroscopy, and powder and single-crystal X-ray diffraction. The molecular structure and the assignment of absorption bands for the obtained compounds were confirmed by quantum chemical calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Coordination Chemistry
化学-无机化学与核化学
CiteScore
2.40
自引率
15.80%
发文量
85
审稿时长
7.2 months
期刊介绍:
Russian Journal of Coordination Chemistry is a journal that publishes reviews, original papers, and short communications on all aspects of theoretical and experimental coordination chemistry. Modern coordination chemistry is an interdisciplinary science that makes a bridge between inorganic, organic, physical, analytical, and biological chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


