从普通中间体对映选择性地获得带有 C4a 或 C8a 季立体中心的十氢喹啉 全合成 (-)-Myrioxazine A
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
(R)-苯甘二醇衍生的过氢恶唑喹啉2为角取代的对映纯十氢喹啉(DHQs)提供了立体选择性途径。2与合适的格氏试剂反应,生成带有C8a杂氮-季立体中心的顺式dhq,而与Michael受体反应,通过还原去除手性诱导剂的2-苯乙醇,生成带有C4a全碳季立体中心的顺式或反式dhq,这取决于用于裂解恶唑烷C-O键的氢化物。理论研究已经阐明了2与迈克尔受体反应机理的复杂性,为正确理解所观察到的立体选择性提供了论据。最后,2与甲醛的反应是可逆的,根据反应温度的不同,可以选择性地形成角取代的羟甲基衍生物12或c8取代的衍生物13。报道了一种从13中快速合成的多核神经元型生物碱(−)-多核恶嗪A。本文章由计算机程序翻译,如有差异,请以英文原文为准。
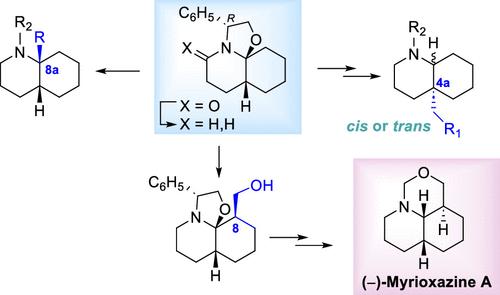
Enantioselective Access to Decahydroquinolines Bearing a C4a or C8a Quaternary Stereocenter from a Common Intermediate Total Synthesis of (−)-Myrioxazine A
(R)-Phenylglycinol-derived perhydrooxazoloquinoline 2 provides stereoselective access to angularly substituted enantiopure decahydroquinolines (DHQs). Reaction of 2 with appropriate Grignard reagents leads to cis-DHQs bearing a C8a aza-quaternary stereocenter, whereas reaction of 2 with Michael acceptors followed by reductive removal of the 2-phenylethanol of the chiral inductor gives rise to cis- or trans-DHQs bearing a C4a all-carbon quaternary stereocenter depending on the hydride used for the cleavage of the oxazolidine C–O bond. Theoretical studies have clarified the mechanistic intricacies of the reaction of 2 with Michael acceptors, providing arguments for a proper understanding of the observed stereoselectivity. Finally, the reaction of 2 with formaldehyde is reversible, allowing the regioselective formation of either the angularly substituted hydroxymethyl derivative 12 or the C8-substituted derivative 13 depending on the reaction temperature. An expeditious synthesis of the Myrioneuron-type alkaloid (−)-myrioxazine A from 13 is reported.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


