通过金属-酚络合增强金属离子的免疫活性
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属离子的免疫调节特性有助于预防和治疗各种疾病的疫苗接种和免疫疗法(即金属免疫疗法)。开发一种可行的方法,使金属离子易于加入疫苗制剂中,并使其具有可控的药代动力学和靶向能力,这是一项正在进行的努力。在此,我们报告了一种简单而高效的金属-酚类组装方法,通过将卵清蛋白(一种模型抗原)和免疫反应性金属离子(即AlIII和MnII)固定在生物相容性配位网络中,在温和条件下形成金属-酚类网络疫苗(mpnv)。mpnv在小鼠皮下和肌肉注射后表现出特异性淋巴结积聚,并引起体液和细胞免疫反应。用MPNVs免疫的小鼠保持了至少10周的强抗体应答,与商业铝佐剂相当。装配方法的模块化提供了双金属掺入MPNVs (MPNVMn+Al),与OVA、AlIII和MnII(即OVA +Al + Mn)的混合物相比,其免疫应答放大高达11倍。此外,MPNVs在抑制小鼠B16F10黑色素瘤的发展中显示出有效的抗癌特性。具体而言,与OVA +Al + Mn治疗相比,MPNVMn+Al治疗导致皮下肿瘤体积减少5倍,转移结节数量减少6.6倍。这项工作为金属有机材料的免疫活性提供了见解,为基于这些材料的疫苗和治疗平台的合理设计奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
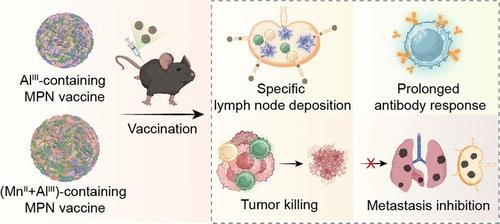
Amplifying the Immune Activity of Metal Ions through Metal–Phenolic Complexation
The immune-modulatory properties of metal ions have contributed to vaccination and immunotherapy (i.e., metalloimmunotherapy) for the prevention and treatment of various diseases. Developing an enabling approach that can readily incorporate metal ions in vaccine formulations and deliver them with controllable pharmacokinetics and targeting ability is an ongoing endeavor. Herein, we report a simple and highly effective metal–phenolic assembly approach, whereby both ovalbumin (a model antigen) and immune-responsive metal ions (i.e., AlIII and MnII) are immobilized within a biocompatible coordination network to form metal–phenolic network vaccines (MPNVs) under mild conditions. The MPNVs demonstrated specific lymph node accumulation and elicited humoral and cellular immune responses following their subcutaneous and intramuscular administration in mice. Mice immunized with MPNVs maintained a robust antibody response for at least 10 weeks, comparable to a commercial aluminum adjuvant. The modularity of the assembly approach afforded dual-metal incorporation into MPNVs (MPNVMn+Al), which amplified immune responses up to 11-fold compared to the mixture of OVA, AlIII, and MnII (i.e., OVA + Al + Mn). Moreover, MPNVs showed effective anticancer properties in suppressing the development of B16F10 melanoma in mice. Specifically, treatment with MPNVMn+Al led to a 5-fold reduction in subcutaneous tumor volume and a 6.6-fold decrease in metastatic nodule number, compared to treatment with OVA + Al + Mn. This work provides insights into the immune activity of metal–organic materials, underpinning the rational design of vaccine and therapeutic platforms based on these materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


