环丙烯基硼酸酯与烯丙基溴化物的区域选择性α-烯丙基化
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-03-31
DOI:10.1021/acs.joc.5c0013510.1021/acs.joc.5c00135
引用次数: 0
摘要
烯丙基硼化合物是在有机化合物中引入烯丙基基团的有价值的亲核试剂。虽然已经报道了许多γ-选择性功能化的例子,例如羰基化合物的烯丙基硼化,但涉及烯丙基硼酸盐的α-选择性碳-碳键形成仍然有限。本研究以烯丙基溴化物为碳基亲电试剂,实现了环丙基硼酸酯α-选择性烯丙基化。环丙烯基硼酸酯与烯丙基溴化物反应,产率中高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
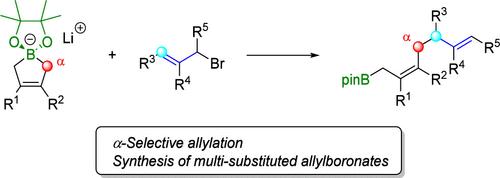
Regioselective α-Allylation of Cyclic Allylborates with Allylic Bromides
Allylic boron compounds are valuable nucleophiles for introducing allylic moieties into organic compounds. While numerous examples of γ-selective functionalization, such as the allylboration of carbonyl compounds, have been reported, α-selective carbon–carbon bond formation involving allylborates remains limited. In this study, α-selective allylation of cyclic allylborates was achieved using allylic bromides as carbon-based electrophiles. The reaction between cyclic allylborates and allylic bromides produced various allylboronates in moderate to good yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


