巯基生物基两性离子表面活性剂中β-羟基提高了其界面活性的耐热性
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
亲水头结构对生物基两性离子表面活性剂的界面性能有显著影响。在各种亲水性基团中,羟基对界面活性的影响相当大。然而,相关机制仍有待阐明。为了研究这一点,本文以生物质油酸甲酯为原料合成了两种新型生物两性离子表面活性剂——苯甲醚基油酰胺乙基羟丙基磺酰基季铵盐(AEHSQA)和苯甲醚基油酰胺乙基磺酰基丙基季铵盐(AESPQA),并对这两种表面活性剂的界面性能进行了评价。结果表明,在温度高达120℃、Ca2+浓度高达2500 mg/L且多一个β-羟基的条件下,AEHSQA能将原油与地下水之间的界面张力降低至10-2 mN/m的超低水平,而AESPQA的耐受性仅为90℃和250 mg/L。采用分子动力学模拟(MDS)方法研究了不同温度和盐度条件下表面活性剂分子在油水界面的界面行为。MDS结果表明,引入羟基可以通过抵抗温度和盐度升高引起的表面活性剂分子亲水性下降和界面松动,提高两性离子表面活性剂的耐热性和耐盐性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
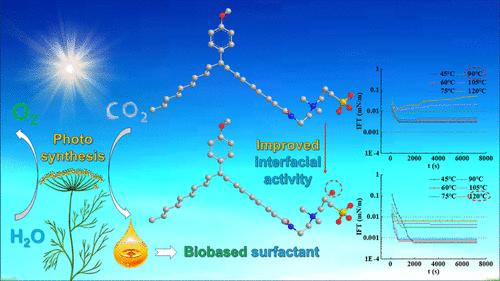
β-Hydroxyl Group in the Sulfonic Biobased Zwitterionic Surfactant Improves Its Thermal Tolerance of Interfacial Activity
The hydrophilic head structure can significantly influence the interfacial performance of biobased zwitterionic surfactants. Among various hydrophilic groups, the hydroxyl group plays a considerable role in the influence on interfacial activity. However, the relevant mechanism remains to be elucidated. To study this point, in this work two new biobased zwitterionic surfactants, anisole-based oleamide ethyl hydroxypropyl sulfonyl quaternary ammonium salt (AEHSQA) and anisole-based oleamide ethyl sulfonylpropyl quaternary ammonium salt (AESPQA), were synthesized from biomass material methyl oleate, and the interfacial performances of the two surfactants were evaluated. It showed that AEHSQA can lower the interfacial tensions between crude oil and groundwater to the ultralow level (<10–2 mN/m) at a temperature of up to 120 °C and a concentration of Ca2+ of up to 2500 mg/L with one more β-hydroxyl group, while the corresponding tolerances of AESPQA are only 90 °C and 250 mg/L. Molecular dynamics simulation (MDS) was employed to study the interfacial behaviors of surfactant molecules at the oil–water interface under conditions of different temperatures and salinities. The results of MDS implied that introducing a hydroxyl group could improve the thermal resistance and salt tolerance of zwitterionic surfactants via resisting the hydrophilicity decline and interfacial looseness of surfactant molecules resulting from the increases in temperature and salinity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


