非多孔和多孔水凝胶的复合电导率谱
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本文报道了两种水凝胶的复合电导率谱。第一种是无孔交联聚丙烯酰胺(PAAm),将其样品浸入浓度高达100 mmol L-1的氯化钾电解质中。将这些光谱与理想电解质溶液的Hollingsworth和Saville理论进行比较,突出了显著的差异;这些暂时归因于阻碍离子迁移和/或离子结合,这赋予了聚电解质的特性。第二种是泡沫黄原胶(XG)溶液,由共存的聚丙烯酰胺(PAAm)网络凝胶化。这些XG-PAAm水凝胶是非常柔顺的多孔聚电解质,可以承受大的压缩应变ϵx,并用于解释电导率和相对介电常数的基础上的空隙率φ和纵横比γ随ϵx变化。在这里,ϕ从初始值ϕ = ϕ≈0.77(其中孔洞是由连通球体组成的密集网络)减小到ϕ→0为ϵx→ϕ0≈0.77的极限。连续介质理论很好地描述了嵌入导电介质和介电介质中的气体球体的数据。然而,尽管电导率谱与理想电解质溶液具有质的相似性,但建立这种相似性所需的有效离子迁移率和浓度却截然不同。PAAm和XG-PAAm水凝胶的阻抗分析提供了定量和定性的证据,证明水凝胶(即使是传统理想和不带电的水凝胶)中的离子传输与理想电解质中的离子传输明显不同。这些见解可以转化为解释复杂的生物介质,与生物电阻抗分析和亚细胞相分离域的研究有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
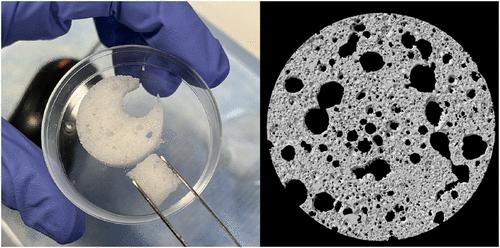
Complex Conductivity Spectra of Nonporous and Porous Hydrogels
This paper reports complex conductivity spectra of two types of hydrogels. The first is a nonporous cross-linked polyacrylamide (PAAm), samples of which are immersed in KCl electrolytes with concentrations up to 100 mmol L–1. Comparing these spectra with the theory of Hollingsworth and Saville for ideal electrolyte solutions highlights notable differences; these are tentatively attributed to hindered ion mobility and/or ion binding that imparts polyelectrolyte characteristics. The second is a foamed xanthan gum (XG) solution that is gelled by a coexisting polyacrylamide (PAAm) network. These XG-PAAm hydrogels are remarkably compliant, porous polyelectrolytes, which can be subjected to large compressive strain ϵx, and used to interpret the conductivity and relative dielectric permittivity on the basis of the void fraction ϕ and aspect ratio γ varying with ϵx. Here, ϕ decreases from the initial value ϕ = ϕ0 ≈ 0.77, where the voids are a dense network of connected spheres, to the limit in which ϕ → 0 as ϵx → ϕ0 ≈ 0.77. The data are very well described by continuum theories for gas spheroids embedded in conducting and dielectric media. However, despite the conductivity spectra bearing qualitative similarities to those of ideal electrolyte solutions, the effective ion mobilities and concentrations required to establish such parallels are profoundly different. Together, the impedance analysis of PAAm and XG-PAAm hydrogels provides quantitative and qualitative evidence that ion transport in hydrogels─even conventionally ideal and uncharged hydrogels─is distinctly different from their ideal electrolyte counterparts. These insights may translate to interpreting complex biological media, pertinent to bioelectrical impedance analysis and studies of subcellular phase-separated domains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


