从退役磷酸铁锂电池粉中焙烧-氨基磺酸水浸提锂铁的反应机理
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
研究了从退役磷酸铁锂(LiFePO4)电池粉末中提取锂和铁的新工艺。将脱碳后的LiFePO4电池粉与氨基甲酸混合,焙烧后用水浸出。采用TG-DSC、XRD、XPS、SEM等方法对焙烧过程中发生的物理化学变化进行了表征。结果表明:焙烧过程中存在质量损失,同时存在吸热现象。许多结块的颗粒附着在焙烧产品的表面。Li以Li2SO4的形式存在,Fe以Fe2(SO4)3、Fe、Fe3O4的形式存在,Al以Al、AlPO4的形式存在。XPS分析表明,有价金属元素(Fe和Al)的价态发生了变化。将脱碳LiFePO4电池粉与氨基磺酸按1:0.77的比例混合,在400℃下焙烧1 h,然后用水浸出。Li、Fe、Al、Cu的提取率分别为99.89%、73.53%、0%、0%。本文章由计算机程序翻译,如有差异,请以英文原文为准。

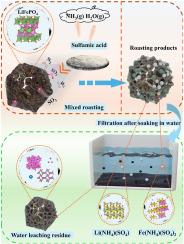
Reaction mechanism of extracting Li and Fe from retired lithium iron phosphate battery powder through roasting and water leaching with sulfamic acid
In this paper, a new process for extracting Li and Fe from retired lithium iron phosphate (LiFePO4) battery powder was studied. The decarbonized LiFePO4 battery powder was mixed with sulfamic acid, roasted, and then leached with water. The physical and chemical changes that occurred during the roasting process were characterized using TG-DSC, XRD, XPS, and SEM methods. The results indicated that mass loss was occurred during the roasting process, accompanied by heat absorption. Many agglomerated particles adhered to the surface of the roasted product. Li was found in the form of Li2SO4, Fe was present as Fe2(SO4)3, Fe, Fe3O4, and Al was detected as Al, AlPO4. XPS analysis revealed that the valence states of valuable metal elements (Fe and Al) had changed. The decarbonized LiFePO4 battery powder was mixed with sulfamic acid in a ratio of 1:0.77, roasted at 400 °C for 1 h, and then leached with water. The extraction rates of Li, Fe, Al, and Cu were 99.89 %, 73.53 %, 0 %, and 0 %, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


