1,3-二恶唑烷-4-酮二元有机催化聚合的高等规聚乳酸
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
二氧恶酮(DOXs)的开环聚合(ROP)提供了获得功能聚酯的途径,这些聚酯具有传统内酯聚合难以获得的性能。然而,典型的催化剂往往在dox中诱导外聚,限制了等规结晶聚合物的合成。本研究报道了一个二元有机催化体系,该体系可以在温和的条件下控制手性2,2,5-三甲基-1,3-二恶唑-4-酮的ROP,通过氢键激活单体和引发剂,有效地减少了外映反应。该策略有利于合成立体规则参数为0.92的高等规聚乳酸(PLA)。此外,共混对映体PLA链形成结晶立体配合物,熔融温度高达195.1℃。这些发现强调了合成立体规则聚酯的可持续和可扩展的方法,为先进材料的应用铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
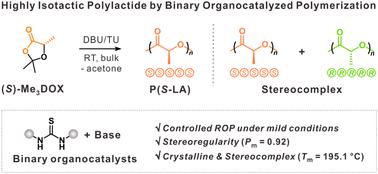
Highly isotactic polylactide by binary organocatalyzed polymerization of 1,3-dioxolan-4-ones†
Ring-opening polymerization (ROP) of dioxolanones (DOXs) provides access to functional polyesters with properties that are challenging to achieve via traditional polymerization of lactones. However, typical catalysts often induce epimerization in DOXs, limiting the synthesis of isotactic, crystalline polymers. This study reports a binary organocatalytic system that enables controlled ROP of chiral 2,2,5-trimethyl-1,3-dioxolan-4-ones under mild conditions, effectively minimizing epimerization by activating both the monomer and initiator via hydrogen bonding. This strategy facilitates the synthesis of highly isotactic poly(lactic acid) (PLA) with a stereoregularity parameter of 0.92. Moreover, blending enantiomeric PLA chains form a crystalline stereocomplex with a high melting temperature of 195.1 °C. These findings highlight a sustainable and scalable approach for synthesizing stereoregular polyesters, paving the way for advanced material applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


