印染木吸附材料对Cr(VI)水溶液的吸附及生态毒理学研究。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
从 Inga edulis 树皮中提取的吸附剂被开发用于去除六价铬离子。用硫酸进行化学活化后,生物质的理化性质发生了显著变化。在 pH 值为 2.0、吸附量分别为 1.0 和 0.25 g L-1 时,IB(莺歌生物质)和 AIB(活化莺歌生物质)吸附材料的去除率较高。吸附动力学可以用 Elovich 模型进行拟合,表明吸附发生在异质表面上。IB 和 AIB 的最大吸附容量分别为 46.0 mg g-1 和 356.6 mg g-1,其吸附行为可分别用 Langmuir(单层)和 Freundlich(多层)模型来描述。XPS 分析证实,由于与含氧官能团的相互作用,Cr(VI) 被还原成了 Cr(III)。热力学评估表明,吸附是自发的,IB 具有放热特性,AIB 具有内热特性。使用水蚤进行的生态毒理学试验表明,4.34 μg L-1 的六价铬浓度会导致 50.0% 的不移动,而材料的吸附作用则消除了毒性,这表明吸附剂在减少环境影响方面非常有效。此外,由 AIB(CPEAIB-Cr-ads-active)吸附六价铬得到的电极在氢进化反应(HER)中表现良好,具有高电流密度和低过电位。该电极的结构具有较高的比表面积,并存在孔隙和空穴,有利于电化学催化,这证明了它在可再生能源和环境解毒方面的应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
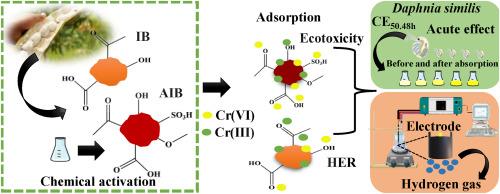
Adsorption and ecotoxicology studies with aqueous solution of Cr(VI) ions using adsorbent materials derived from Inga edulis
Adsorbents derived from the bark of Inga edulis were developed for the removal of Cr(VI) ions. Chemical activation with sulfuric acid led to significant changes in the physicochemical properties of the biomass. The adsorbent materials denoted IB (inga biomass) and AIB (activated inga biomass) showed higher removal efficiencies at pH 2.0 and dosages of 1.0 and 0.25 g L−1, respectively. The adsorption kinetics could be fitted using the Elovich model, indicating that the adsorption occurred on heterogeneous surfaces. The maximum adsorption capacities were 46.0 mg g−1 for IB and 356.6 mg g−1 for AIB, with behaviors that could be described by the Langmuir (monolayer) and Freundlich (multilayer) models, respectively. XPS analyses confirmed the reduction of Cr(VI) to Cr(III), due to interactions with oxygenated functional groups. Thermodynamic evaluation indicated that the adsorption was spontaneous, with exothermic character for IB and endothermic character for AIB. Ecotoxicological assays using Daphnia similis showed that a Cr(VI) concentration of 4.34 μg L−1 caused 50.0 % immobility, while adsorption by the materials eliminated the toxicity, demonstrating the effectiveness of the adsorbents in reducing environmental impacts. Additionally, an electrode derived from the adsorption of Cr(VI) on AIB, denoted CPEAIB-Cr-ads-active, presented good performance in the hydrogen evolution reaction (HER), with high current density and low overpotential. The structure of the electrode, with high surface area and the presence of pores and cavities, was favorable for electrochemical catalysis, evidencing its potential for use in applications concerning renewable energy and environmental detoxification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


