Co纳米粒子与CoN位点协同催化氨分解产氢的研究
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
设计了一系列碳基Co催化剂用于氨分解制氢。值得注意的是,CoCe-NC催化剂表现出优异的活性,在600℃条件下实现了NH3的完全分解,产氢率达到1840 mmol·gcat−1·h−1。采用XRD、Raman、XPS、EXAFS、Co - tpd等先进表征技术分析了催化剂表面Co的形态。存在于Co碳质催化剂表面的活性物质包括CoN、Co纳米颗粒(NPs)和较大的金属体。金属有机骨架(MOF)前驱体的约束在Co NPs的形成中起着至关重要的作用。此外,碳质衬底中N和Ce的掺杂有效地促进了Co NPs和CoN结构的形成。通过DFT模拟了不同活性物质对氨分解的影响,验证了Co NPs为最优活性位点。此外,还发现自催化能力相对较差的CoN与Co NPs协同催化氨分解,有效降低了N2和H2复合的能垒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
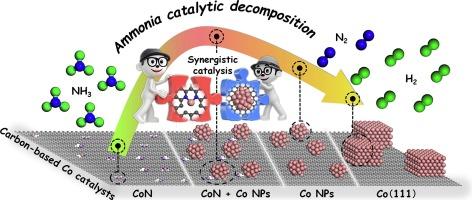
Synergistic catalysis of CoN sites and Co nanoparticles for efficient COx-free hydrogen production from ammonia decomposition
A series of carbon-based Co catalysts was designed for hydrogen production from ammonia decomposition. Notably, the CoCe-NC catalyst exhibited the superior activity, achieving complete NH3 decomposition at 600 °C with a remarkable hydrogen production rate of 1,840 mmol·gcat−1·h−1. Advanced characterization techniques, including XRD, Raman, XPS, EXAFS, and CO-TPD, were employed to analyze the surface Co species of these catalysts. The active species present on the Co carbonaceous catalyst surface include CoN, Co nanoparticles (NPs), and larger metallic bulk. The confinement of metal–organic framework (MOF) precursor plays a crucial role in the formation of Co NPs. Moreover, it was found that N and Ce doping in the carbonaceous substrate effectively promoted the formation of Co NPs and CoN structures. DFT simulations of the ammonia decomposition behaviors upon various active species were conducted, verifying that Co NPs serve as the optimal active sites. Furthermore, it was discovered that CoN, with relatively poor self-catalytic ability, synergistically catalyzed the ammonia decomposition with Co NPs, effectively decreasing the energy barriers for recombination of both N2 and H2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


