免疫检查点TIM-3调节小胶质细胞和阿尔茨海默病
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
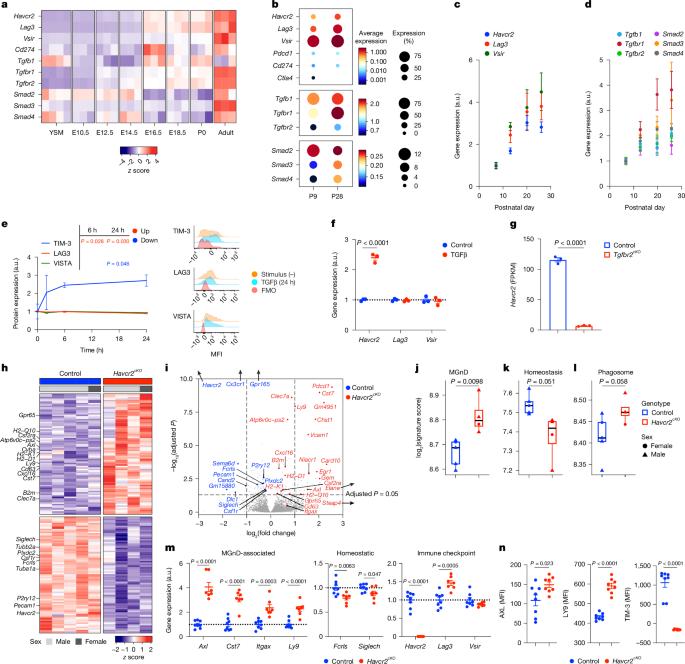
小胶质细胞是大脑中的常驻免疫细胞,在神经发育和神经炎症中起着关键作用1,2。本研究探讨了免疫检查点分子TIM-3(由HAVCR2编码)在小胶质细胞中的功能。TIM-3最近被确定为迟发性阿尔茨海默病的遗传风险因素,它可以诱导T细胞衰竭。然而,其在脑小胶质细胞中的具体功能尚不清楚。我们在小鼠模型中证明tgf - β信号可诱导小胶质细胞中TIM-3的表达。反过来,TIM-3通过其羧基末端尾部与SMAD2和TGFBR2相互作用,通过促进tgfbr介导的SMAD2磷酸化来增强TGFβ信号传导,这一过程维持小胶质细胞的稳态。小胶质细胞中Havcr2基因缺失导致吞噬活性增加,基因表达谱与神经退行性小胶质细胞表型(MGnD)一致,也被称为疾病相关小胶质细胞(DAM)。此外,小胶质细胞靶向缺失Havcr2可改善5×FAD小鼠(阿尔茨海默病的转基因模型)的认知障碍并减少淀粉样蛋白-β病理。单核RNA测序显示,在havcr2缺陷5×FAD小鼠中存在MGnD小胶质细胞亚群,其特征是促吞噬和抗炎基因表达增加,促炎基因表达减少。在havcr2缺陷5×FAD小鼠中,大多数小胶质细胞簇的单细胞RNA测序数据证实了这些转录组变化。我们的研究结果揭示了TIM-3通过TGFβ信号介导小胶质细胞稳态,并强调了靶向小胶质细胞TIM-3治疗阿尔茨海默病的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Immune checkpoint TIM-3 regulates microglia and Alzheimer’s disease
Microglia are the resident immune cells in the brain and have pivotal roles in neurodevelopment and neuroinflammation1,2. This study investigates the function of the immune-checkpoint molecule TIM-3 (encoded by HAVCR2) in microglia. TIM-3 was recently identified as a genetic risk factor for late-onset Alzheimer’s disease3, and it can induce T cell exhaustion4. However, its specific function in brain microglia remains unclear. We demonstrate in mouse models that TGFβ signalling induces TIM-3 expression in microglia. In turn, TIM-3 interacts with SMAD2 and TGFBR2 through its carboxy-terminal tail, which enhances TGFβ signalling by promoting TGFBR-mediated SMAD2 phosphorylation, and this process maintains microglial homeostasis. Genetic deletion of Havcr2 in microglia leads to increased phagocytic activity and a gene-expression profile consistent with the neurodegenerative microglial phenotype (MGnD), also referred to as disease-associated microglia (DAM). Furthermore, microglia-targeted deletion of Havcr2 ameliorates cognitive impairment and reduces amyloid-β pathology in 5×FAD mice (a transgenic model of Alzheimer’s disease). Single-nucleus RNA sequencing revealed a subpopulation of MGnD microglia in Havcr2-deficient 5×FAD mice characterized by increased pro-phagocytic and anti-inflammatory gene expression alongside reduced pro-inflammatory gene expression. These transcriptomic changes were corroborated by single-cell RNA sequencing data across most microglial clusters in Havcr2-deficient 5×FAD mice. Our findings reveal that TIM-3 mediates microglia homeostasis through TGFβ signalling and highlight the therapeutic potential of targeting microglial TIM-3 in Alzheimer’s disease. The immune-checkpoint molecule TIM-3 regulates microglial homeostasis, and its microglial-specific deletion reduced cognitive impairment in a mouse model of Alzheimer’s disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


