跨越小鼠视觉皮层多个区域的功能连接组学
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
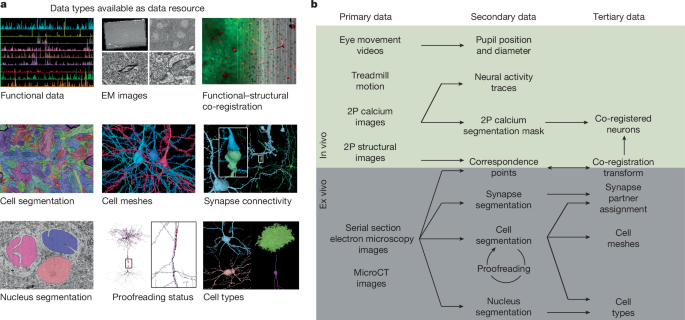
了解大脑需要了解神经元对形成它们的电路结构的功能反应。在这里,我们介绍了MICrONS功能连接组数据集,该数据集具有在清醒的小鼠中观看自然和合成刺激的初级视觉皮层(VISp)和高级视觉区域(VISrl, VISal和VISlm)中约75,000个神经元的密集钙成像。这些数据与包含超过20万个细胞和5亿个突触的电子显微镜重建共同记录。对神经元子集的校对产生了重建,包括完整的树突树以及局部和区域间的轴突投影,这些轴突投影映射了每个神经元数千个细胞间的连接。该数据集作为开放获取资源发布,包括用于数据检索和分析的工具1,2。相关研究描述了其用于细胞类型的综合表征3,4,5,6,皮质柱的突触水平连接图4,以及揭示与基因表达数据相关的细胞类型特异性抑制连接4,7。在功能上,我们确定了信息如何跨视觉空间整合的新计算原理,表征了新型的神经元不变性,并将结构和功能结合在一起,揭示了区域内和区域间兴奋性神经元之间连接的一般原理。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Functional connectomics spanning multiple areas of mouse visual cortex
Understanding the brain requires understanding neurons’ functional responses to the circuit architecture shaping them. Here we introduce the MICrONS functional connectomics dataset with dense calcium imaging of around 75,000 neurons in primary visual cortex (VISp) and higher visual areas (VISrl, VISal and VISlm) in an awake mouse that is viewing natural and synthetic stimuli. These data are co-registered with an electron microscopy reconstruction containing more than 200,000 cells and 0.5 billion synapses. Proofreading of a subset of neurons yielded reconstructions that include complete dendritic trees as well the local and inter-areal axonal projections that map up to thousands of cell-to-cell connections per neuron. Released as an open-access resource, this dataset includes the tools for data retrieval and analysis1,2. Accompanying studies describe its use for comprehensive characterization of cell types3–6, a synaptic level connectivity diagram of a cortical column4, and uncovering cell-type-specific inhibitory connectivity that can be linked to gene expression data4,7. Functionally, we identify new computational principles of how information is integrated across visual space8, characterize novel types of neuronal invariances9 and bring structure and function together to uncover a general principle for connectivity between excitatory neurons within and across areas10,11. Dense calcium imaging combined with co-registered high-resolution electron microscopy reconstruction of the brain of the same mouse provide a functional connectomics map of tens of thousands of neurons of a region of the primary cortex and higher visual areas.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


