萘啶酮衍生物作为具有抗肿瘤功效的选择性强效PKMYT1抑制剂的发现
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
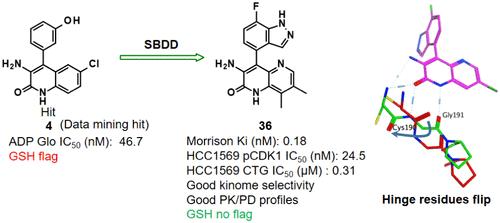
PKMYT1是细胞周期的关键调节因子,特别是通过抑制CDK1磷酸化参与G2/M转变,是癌症治疗的一个有希望的治疗靶点。在Roche kinome筛选数据库中进行数据挖掘,发现在10 μM浓度下具有100% PKMYT1抑制活性的hit,并通过PKMYT1酶测进一步验证,显示出两位数的纳摩尔效价。该hit具有喹啉酮中心核心和苯酚头基。用茚唑部分取代有问题的酚头基团,导致激酶铰链半胱氨酸和甘氨酸残基的翻转,从而产生一系列具有增强效力,优越的激酶组选择性和无谷胱甘肽标志的衍生物。进一步的结构微调导致化合物36的发现,这是一种新的,选择性的,有效的PKMYT1抑制剂,具有良好的口服药代动力学特征和有希望的体内抗肿瘤功效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of Naphthyridinone Derivatives as Selective and Potent PKMYT1 Inhibitors with Antitumor Efficacy
PKMYT1 is a crucial regulator of the cell cycle, particularly involved in the G2/M transition through the inhibitory phosphorylation of CDK1, and is a promising therapeutic target for cancer therapy. Data mining in the Roche kinome screen database identified a hit characterized by 100% PKMYT1 inhibitory activity at a 10 μM concentration, which was further validated with a PKMYT1 enzymatic assay showing double-digit nanomolar potency. The hit featured a quinolinone central core and a phenol headgroup. The replacement of the problematic phenol headgroup with an indazole moiety induced a flip in the kinase hinge cysteine and glycine residues, resulting in a series of derivatives with enhanced potency, superior kinome selectivity, and no GSH flag. Further structural fine-tuning led to the discovery of compound 36, a novel, selective, and potent PKMYT1 inhibitor with favorable oral pharmacokinetic profiles and promising in vivo antitumor efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


