三室电解法回收废三元锂离子电池中镍钴合金、二氧化锰、石墨和Li2CO3
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
关键金属的分离和回收对于废锂离子电池的可持续回收至关重要。本文提出了一种三室电解方法,通过施加电场,在一步过程中实现多种组分的直接分离。在中间隔间中,锂镍钴锰氧化物(NCM)材料溶解并从石墨中分离出来。同时,Ni-Co合金在阴极沉积,MnO2在阳极合成。此外,Li+在电解液中通过化学沉淀法浓缩并沉淀为Li2CO3。结果表明,在80℃下,废镍钴锰酸锂(SNCM)材料中Ni、Co、Mn和Li的浸出效率分别为94.30 %、90.78 %、98.19 %和94.33 %,浸出时间为6 h。镍、钴、锰的回收率分别为87.22 %、81.50 %和48.89 %。Ni-Co合金中Ni和Co的含量分别为33.42 %和44.82 %。通过简单的浓缩和沉淀,从电解液中回收纯度大于95 %的Li2CO3。每回收1 kg SNCM电极粉,三室电解和电解液再利用的碳排放量分别为3.81 kg CO2当量和- 3.47 kg CO2当量,经济效益分别为0.25美元和2.06美元。这种方法可以在一个步骤中同时分离和回收多种金属,解决了对关键能源金属日益增长的需求。本文章由计算机程序翻译,如有差异,请以英文原文为准。

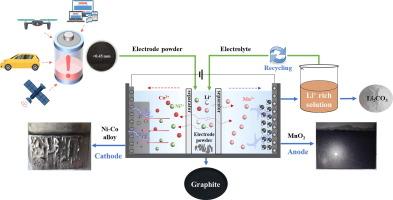
Recovery of Ni-Co alloy, MnO2, graphite and Li2CO3 from spent ternary lithium-ion batteries through three-compartment electrolysis
The separation and recovery of key metals are essential for the sustainable recycling of spent lithium-ion batteries (SLIBs). In this paper, a three-compartment electrolysis is proposed to achieve the direct separation of multiple components in a one-step process through the application of an electric field. In the middle compartment, lithium nickel cobalt manganese oxide (NCM) material dissolves and separates from graphite. Simultaneously, Ni-Co alloy deposits at the cathode, while MnO2 synthesizes at the anode. Additionally, Li+ concentrates and subsequently precipitates as Li2CO3 through chemical precipitation in the electrolyte. As a result, the leaching efficiencies of Ni, Co, Mn, and Li in spent lithium nickel cobalt manganese oxide (SNCM) materials reach 94.30 %, 90.78 %, 98.19 %, and 94.33 %, respectively, in the middle compartment within 6 h at 80 ℃. Meanwhile, the recovery rates for Ni, Co, and Mn are 87.22 %, 81.50 %, and 48.89 %, respectively. The proportions of Ni and Co in the Ni-Co alloy are 33.42 % and 44.82 %, respectively. Li2CO3 with a purity greater than 95 % is recovered from the electrolyte through simple concentration and precipitation. For every 1 kg of SNCM electrode powder recovered, the carbon emissions from the three-compartment electrolysis and electrolyte reuse are 3.81 kg CO2 eq. and −3.47 kg CO2 eq., resulting in economic benefits of $0.25 and $2.06, respectively. This approach enables the simultaneous separation and recovery of multiple metals in a single step, addressing the growing demand for critical energy metals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


