CuO-ZnO-TiO2催化氧化异丙烯制过氧化氢异丙烯的应用、表征及模拟
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
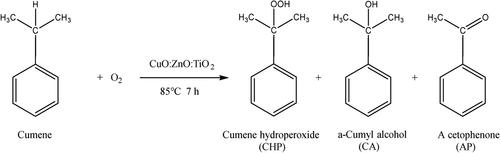
采用超声辅助共沉淀法制备CuO-ZnO-TiO2。研究了其在氧液相氧化异丙苯制过氧化异丙苯(CHP)中的催化性能。在CuO:ZnO:TiO2的摩尔比为3:1:1.33,进料比(催化剂/异丙苯)为7.5 mg/mL,反应温度为85℃,反应时间为7 h,氧气流量为15 mL/min的条件下,异丙苯的转化率为37.2%,CHP的选择性为94.5%。通过XRD、SEM、TEM、EDS、N2吸附/脱附等表征表明,3CuO-ZnO-TiO2被浓缩,粒径约为50 nm,比面积约为110 m2/g,孔体积为0.0048 cm3/g,活性成分高度分散。XPS和O2-TPD表征表明,3CuO-ZnO-TiO2中含有大量的晶格氧和活性氧。DFT模拟表明,ROO·更容易生成,更容易与CuO-ZnO表面结合,促进异丙烯氧化成异丙烯过氧化物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Application, Characterization, and Simulation of CuO-ZnO-TiO2 for Catalytic Oxidation of Cumene to Cumene Hydroperoxide
CuO-ZnO-TiO2 was prepared by ultrasound-assisted coprecipitation. Its catalytic performance in the liquid-phase oxidation of cumene with oxygen to cumene hydroperoxide (CHP) was studied. Under the reaction conditions of CuO:ZnO:TiO2 molar ratio of 3:1:1.33, feed ratio (catalyst/cumene) of 7.5 mg/mL, reaction temperature of 85 °C, reaction time of 7 h, and oxygen flow rate of 15 mL/min, the conversion of cumene was 37.2% and the selectivity of CHP was 94.5%. Characterization by XRD, SEM, TEM, EDS, and N2 adsorption/desorption showed 3CuO-ZnO-TiO2 was concentrated with a particle size of about 50 nm, specific area of about 110 m2/g with a pore volume of 0.0048 cm3/g, and high dispersion of active components. XPS and O2-TPD characterization indicated that 3CuO-ZnO-TiO2 contains a large amount of lattice oxygen and reactive oxygen species. DFT simulation indicated ROO· is more easily generated and more likely to bind to the CuO-ZnO surface to facilitate oxidation of cumene to cumene peroxides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


