可见光介导的亚甲基亚胺脱羧(氨基)烷基化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报道了一种高效的,有机光催化的亚甲基亚胺脱羧(氨基)烷基化反应,使用易得的羧酸作为烷基化剂。这种转化表现出广泛的范围,各种羧酸,包括甘氨酸衍生物,被用作自由基前体。使用4CzIPN作为光催化剂也允许非苯基的仲羧酸和叔羧酸的应用,克服了以前的限制。非预官能化前体的广泛适用性和温和的条件是该方法的亮点。通过自由基俘获实验确定了关键自由基中间体的中间性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
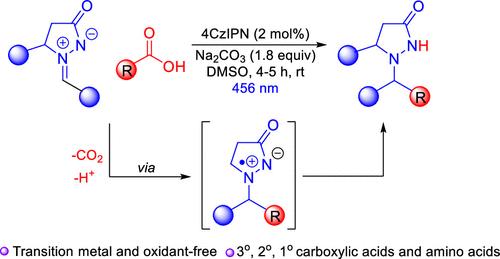
Visible-Light-Mediated Decarboxylative (Amino)Alkylation of Azomethine Imines
Herein, we report an efficient, organophotocatalyzed decarboxylative (amino)alkylation of azomethine imines using readily available carboxylic acids as alkylating agents. This transformation exhibits wide scope, and a variety of carboxylic acids, including glycine derivatives, were employed as radical precursors. The use of 4CzIPN as the photocatalyst allowed the application of nonbenzylic secondary and tertiary carboxylic acids also, overcoming previous limitations. The wide scope, applicability of nonprefunctionalized precursors, and mild conditions are the highlights of this method. The intermediacy of key radical intermediates was established by radical trapping experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


