揭示Bi2O3纳米结构ph依赖性催化CO2电化学还原性能的来源
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
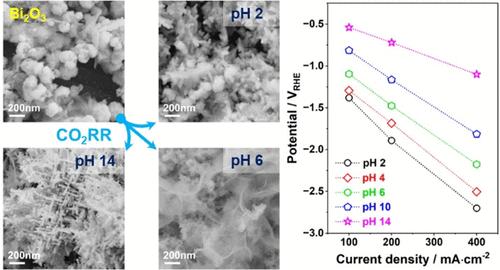
在过去的十年中,将二氧化碳转化为有价值的化学品和燃料的电化学转化作为一种通向碳中和循环经济的有希望的途径引起了越来越多的兴趣。本研究研究了Bi2O3气体扩散电极(Bi-GDEs)用于将CO2转化为甲酸/甲酸(HCOOH/HCOO -),该电极在高电流密度下具有优异的选择性。该催化剂是通过一锅微波辅助工艺合成的,该工艺快速,节能,可扩展,利用绿色溶剂乙二醇。所得的Bi2O3纳米结构实现了CO2转化的近单位选择性,在较宽的pH范围内形成HCOOH/HCOO -的法拉第效率超过95%。催化活性强烈依赖于pH值,在给定的电流密度下,pH值的增加会降低过电位。为了阐明这种ph依赖性活性的来源,进行了operando拉曼光谱,死后扫描电子显微镜(SEM)和电双层表征。Operando Raman结果表明,Bi2O3在高酸性或碱性电解质中更容易还原,而在接近中性ph时,其还原受到抑制。然而,在与CO2RR相关的高负电位下,阳离子Bi完全转化为金属Bi。尽管在不同的电解质pH值下结构会发生变化,但金属Bi仍然是活性相,这解释了Bi- gde在很宽的pH范围内的高选择性。在CO2RR条件下,解剖后的SEM图像突出了电解质pH对形态演变的影响。在最高pH值为14时,出现了层次化的枝晶结构,双层电容增加了100%,这表明电化学活性表面积和CO2RR活性显著增强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling the Origin of pH-Dependent Catalytic Performance of Bi2O3 Nanostructure for Electrochemical CO2 Reduction
Over the past decade, the electrochemical conversion of CO2 into valuable chemicals and fuels has garnered increasing interest as a promising pathway toward a carbon-neutral circular economy. This study investigates Bi2O3 gas diffusion electrodes (Bi-GDEs) for the conversion of CO2 to formic acid/formate (HCOOH/HCOO–), which demonstrate excellent selectivity at high current densities. The catalyst is synthesized through a one-pot microwave-assisted process that is rapid, energy-efficient, and scalable, utilizing the green solvent ethylene glycol. The resulting Bi2O3 nanostructure achieves near-unit selectivity for CO2 conversion, with a faradaic efficiency exceeding 95% for HCOOH/HCOO– formation across a wide pH range. The catalytic activity is strongly pH-dependent, with an increase in the pH reducing the overpotential at a given current density. To elucidate the origin of this pH-dependent activity, operando Raman spectroscopy, post-mortem scanning electron microscopy (SEM), and electrical double layer characterization were performed. Operando Raman results reveal that Bi2O3 undergoes reduction more readily in highly acidic or basic electrolytes, whereas its reduction is inhibited near neutral pH. However, at highly negative potentials relevant to CO2RR, cationic Bi species fully convert to metallic Bi. Despite structural variations at different electrolyte pH values, metallic Bi remains the active phase, explaining the high selectivity of Bi-GDEs across a broad pH range. Post-mortem SEM images highlight the influence of electrolyte pH on morphological evolution under CO2RR conditions. At the highest pH of 14, a hierarchical dendritic structure emerges, showing an increase of 100% in double layer capacitance, which evidences the significant enhancement in the electrochemical active surface area and consequently the CO2RR activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


