Bclaf1介导超增强子驱动的POLR2A激活,增强亚硝胺诱导的食管癌中染色质的可及性
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
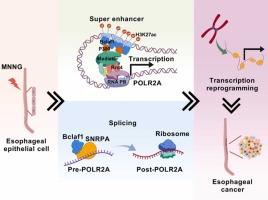
基因-环境相互作用是亚硝胺诱导食管癌发生的关键因素。虽然食管癌(ESCA)的遗传机制是明确的,但表观遗传驱动仍然难以捉摸。本研究确定了以b细胞淋巴瘤-2相关转录因子1 (Bclaf1)为中心的亚硝胺诱导(methylnitronnitrosoguanidine, MNNG)食管癌发生的表观遗传调控新机制。在亚硝胺诱导的恶性转化细胞(MNNG-M)中,Bclaf1的表达随着恶性程度的增加而逐渐升高,Bclaf1水平升高与ESCA患者预后不良相关。在功能上,Bclaf1在体外和体内均能显著促进MNNG-M和ESCA细胞的异常增殖。从机制上看,转座酶可及染色质测序(ATAC-seq)结果表明,Bclaf1沉默显著降低了染色质可及性,从而损害了新转录RNA的合成。Bclaf1激活RNA聚合酶II亚基POLR2A,通过不同的转录和剪接机制促进染色质可及性。更具体地说,靶标切割和标记(CUT&Tag)分析显示,Bclaf1/P300/H3K27ac在POLR2A启动子处共同募集,通过POLR2A超级增强子的E2/E3元件驱动转录。此外,rna结合蛋白免疫沉淀(RIP)实验表明,Bclaf1辅助因子小核核糖核蛋白多肽A (SNRPA)与pre-POLR2A相互作用,调节其剪接。总之,我们的研究表明Bclaf1通过控制POLR2A的转录和剪接活性来促进亚硝胺诱导的ESCA,为早期发现和干预提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bclaf1 Mediates Super-Enhancer-Driven Activation of POLR2A to Enhance Chromatin Accessibility in Nitrosamine-induced Esophageal Carcinogenesis
Gene-environment interactions are pivotal contributors to nitrosamine-induced esophageal carcinogenesis. While genetic mechanisms in esophageal carcinoma (ESCA) are well-defined, epigenetic drivers remain elusive. This study identifies a novel mechanism of epigenetic regulation centered on B-cell lymphoma-2-associated transcription factor 1 (Bclaf1) in nitrosamine-induced (Methylnitronitrosoguanidine, MNNG) esophageal carcinogenesis. In nitrosamine-induced malignant transformation cells (MNNG-M), Bclaf1 expression is progressively increased with malignancy, and elevated Bclaf1 levels are correlated with poor prognosis in ESCA patients. Functionally, Bclaf1 significantly promotes the abnormal proliferation of MNNG-M and ESCA cells in vitro and in vivo. Mechanistically, transposase-accessible chromatin sequencing (ATAC-seq) results suggest that Bclaf1 silencing markedly reduces chromatin accessibility, thereby impairing the synthesis of newly transcribed RNA. Bclaf1 activates RNA polymerase II subunit POLR2A to promote chromatin accessibility through distinct transcriptional and splicing mechanisms. More specifically, cleavage under targets and tagmentation (CUT&Tag) assays revealed Bclaf1/P300/H3K27ac co-recruitment at the POLR2A promoter, driving transcription via the E2/E3 elements of the POLR2A super-enhancer. Additionally, RNA-binding protein immunoprecipitation (RIP) assays demonstrated that the Bclaf1 cofactor, small nuclear ribonucleoprotein polypeptide A (SNRPA), interacts with pre-POLR2A to regulate its splicing. Collectively, our study reveals that Bclaf1 facilitates nitrosamine-induced ESCA by controlling POLR2A transcriptional and splicing activities, providing novel insight for early detection and intervention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


