用于骨肿瘤治疗的 Fe-Ppy@CaO2 改性聚醚醚酮的电子泵和光子捕获效应产生的选择性抗肿瘤作用
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
骨肿瘤死亡率高、致残率高,已成为临床面临的重大挑战。因此,有必要设计骨肿瘤治疗和骨修复材料。在这项工作中,fe掺杂的聚吡咯(Fe-Ppy)和CaO2在磺化聚醚酮(SP)上构建,形成多级响应涂层。该涂层通过化学动力疗法(CDT)、光热疗法(PTT)和联合免疫疗法实现长效抗肿瘤。Fe-Ppy作为电子泵,通过将- nh2 -氧化为- n + -补充Fe2+,在肿瘤微环境(TME)中持续进行Fenton反应并持续产生活性氧(ROS)。CaO2选择性地提供外源H2O2响应TME,促进电子循环。Fe掺杂导致的近红外光吸收增强,多孔结构诱导的散射和折射减弱导致的光子陷阱增加,改善了改性SP的光热转换。此外,持久的ROS和有效的光热转换增强了M1的激活,分泌TNF-α和IFN杀死肿瘤细胞。在肿瘤治疗后,Fe-Ppy@CaO2-modified SP可通过消除TME和NIR刺激,使巨噬细胞自适应转换为M2,促进成骨。总之,Fe-Ppy@CaO2-modified SP具有持久ROS,增强光热转化和免疫调节作用,是骨肿瘤治疗和组织修复的潜在候选物质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
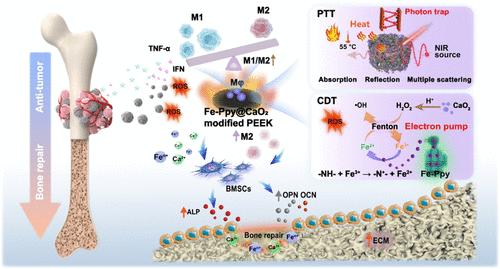
Electron Pump and Photon Trap Effect-Derived Selective Antitumor of Fe-Ppy@CaO2-Modified Polyetheretherketone for Bone Tumor Therapy
Bone tumors with high mortality and disability have become a major clinical challenge. Herewith, it is necessary to design materials for bone tumor therapy and bone repair. In this work, Fe-doped polypyrrole (Fe-Ppy) and CaO2 are constructed on sulfonated polyetheretherketone (SP) to form a multistage-responsive coating. The coating achieves long-lasting antitumor through chemodynamic therapy (CDT), photothermal therapy (PTT), and combined immunotherapy. Fe-Ppy acts as an electron pump to replenish Fe2+ through oxidizing –NH– to –N+–, which lasts the Fenton reaction and persistently produces reactive oxygen species (ROS) in the tumor microenvironment (TME). CaO2 selectively provides exogenous H2O2 in response to TME to boost the electron cycle. Stronger near-infrared light absorption due to Fe doping and more photon traps caused by porous structure-induced scattering and refraction diminishment improve the photothermal conversion of modified SP. Furthermore, long-lasting ROS and effective photothermal conversion enhance M1 activation to secrete TNF-α and IFN to kill tumor cells. After tumor therapy, Fe-Ppy@CaO2-modified SP could adaptively switch the macrophage to M2 and promote osteogenesis with the abolishment of TME and NIR stimulation. In summary, Fe-Ppy@CaO2-modified SP with long-lasting ROS, enhanced photothermal conversion, and immunomodulation is a potential candidate for bone tumor therapy and tissue repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


