CuO-MgO纳米复合材料CO2RR的co2触发击穿和可达高表面积纳米多孔Cu阴极的形成
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
以氧化铜和氧化镁的纳米复合材料(CuO-MgO)为原料,在零间隙CO2还原反应(CO2RR)电解槽中原位制备了可接近的高表面积纳米铜孔电极。在去除MgO后,得到了具有201 m2/g和30 nm规则孔的纳米孔CuO。电化学还原后的铜电极,在水半电池条件下测得的单层表面氧化电荷,其粗糙度系数达到了前所未有的36 cm2Cu/cm2geo。在MEA测试条件下,MgO在水和CO2存在下的现场溶解导致纳米孔铜的形成,从而暴露出大量的三相边界,供质量和电荷载流子使用。铜电极从宏观到纳米尺度的均匀性是获得良好的CO2RR性能和抑制析氢反应的关键。在电池电压为3.4 V时,纳米孔高表面积Cu的最大CO2RR电流密度(jCO2RR)为150.8±4.8 mA/cm2。在3.2 V下,在103.4±9.9 mA/cm2下,C2法拉第效率最高为49.6±6.7%,主要由乙烯产生,法拉第效率为31.0±0.5%。本文章由计算机程序翻译,如有差异,请以英文原文为准。

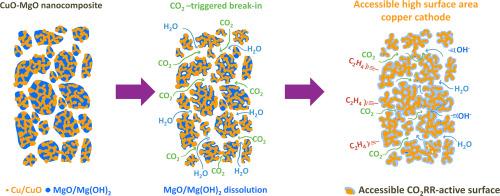
CO2-triggered break-in and formation of accessible high surface area nanoporous Cu cathode for CO2RR from CuO-MgO nanocomposites
Accessible high surface area copper nanoporous electrode is created in a zero-gap CO2 reduction reaction (CO2RR) electrolyzer in-situ from a nanocomposite of copper oxide and magnesium oxide (CuO-MgO). Upon the removal of MgO, a nanoporous CuO exhibiting a very compelling surface area of 201 m2/g with regular 30 nm pores is obtained. After electrochemical reduction, it transformed into a Cu electrode with an unprecedented high roughness factor of 36 cm2Cu/cm2geo based on the monolayer surface oxidation charge measured under aqueous half-cell conditions. Under MEA testing conditions, the on-site dissolution of MgO under the presence of water and CO2 leads to formation of nanoporous copper that exposes large amounts of triple-phase boundaries accessible for mass and charge carriers. The uniformity of the obtained Cu electrode, spanning from macro to nanoscale, is a key essence towards good CO2RR performance and the suppression of hydrogen evolution reaction. The nanoporous high surface area Cu delivers a maximum CO2RR current density (jCO2RR) of 150.8 ± 4.8 mA/cm2 at a cell voltage of 3.4 V. At 3.2 V, it delivered a maximum C2 Faradaic efficiency of 49.6 ± 6.7 % at 103.4 ± 9.9 mA/cm2 that was dominated by ethylene production, exhibiting a Faradaic efficiency of 31.0 ± 0.5 %.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


