揭示工业选择性催化还原(SCR)催化剂的历程:了解失活机制和创新再生技术
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
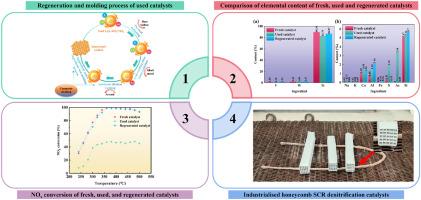
使用V2O5-WO3/TiO2催化剂选择性催化还原(SCR)氮氧化物(NOx)对于减少燃煤电厂的排放至关重要。然而,烟气中的砷和碱金属使催化剂失活是一个重大的操作挑战。本研究的重点是工业SCR催化剂在真实煤燃烧条件下的中毒,具体解决了高砷、高硫环境下的失活机制和再生策略。通过多尺度表征(XPS, TGA, NH3-TPD)和动力学分析,我们证明砷(As2O3/As2O5)和钠(Na)通过(i)阻断Brønsted酸位点,(ii)将V4+氧化为V5+(降低氧化还原活性),以及(iii)形成孔隙堵塞硫酸盐来协同失活催化剂。为了解决这个问题,我们开发了一种新的两步再生方案,结合臭氧氧化(0.5 wt% H2SO4, 120分钟O3暴露)和氨碱性洗涤(1.0 mol/L NH4OH)。再生催化剂被制备成工业规模的蜂窝状单体,以模拟现实世界的操作条件,在380℃下,再生催化剂的as去除率达到97.31%,NOx转化效率达到98.7%,性能与新鲜催化剂相当。最重要的是,再生催化剂在模拟烟气(500 ppm SO2, 10% H2O)下表现出持续的稳定性(符合GB/T 31587-2015标准),与购买新催化剂相比,成本降低了30%。这项工作为煤电系统中的SCR催化剂再生提供了可行的见解,强调了污染物特定化学物质(As与碱金属)和过程驱动的再激活策略之间的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Unveiling the journey of industrial selective catalytic reduction (SCR) catalysts: Understanding deactivation mechanisms and innovating regeneration techniques
The selective catalytic reduction (SCR) of nitrogen oxides (NOx) using V2O5-WO3/TiO2 catalysts is critical for mitigating emissions from coal-fired power plants. However, catalyst deactivation by arsenic (As) and alkali metals in flue gas poses a major operational challenge. This study focuses on industrial SCR catalysts poisoned under real-world coal combustion conditions, specifically addressing deactivation mechanisms and regeneration strategies tailored to this high-arsenic, high-sulfur environment. Through multi-scale characterization (XPS, TGA, NH3-TPD) and kinetic analyses, we demonstrate that arsenic (As2O3/As2O5) and sodium (Na) synergistically deactivate the catalyst by (i) blocking Brønsted acid sites, (ii) oxidizing V4+ to V5+ (reducing redox activity), and (iii) forming pore-clogging sulfates. To counteract this, we developed a novel two-step regeneration protocol combining ozone oxidation (0.5 wt% H2SO4, 120 min O3 exposure) with ammonia alkaline washing (1.0 mol/L NH4OH). Regenerated catalysts were prepared as industrial-scale honeycomb monoliths to emulate real-world operating conditions, achieving 97.31 % As removal and restoring 98.7 % NOx conversion efficiency at 380 °C—performance comparable to fresh catalysts. Crucially, the regenerated catalyst exhibits sustained stability (>15 days, GB/T 31,587–2015 compliance) under simulated flue gas (500 ppm SO2, 10 % H2O), with a 30 % cost reduction versus fresh catalyst procurement. This work provides actionable insights for SCR catalyst regeneration in coal power systems, emphasizing the interplay between contaminant-specific chemistries (As vs. alkali metals) and process-driven reactivation strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


