寻找和回收停滞的剪接体
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
pre-mRNA的剪接需要高保真度和巨大的序列灵活性,不可避免地导致异常的、停滞的复合体必须被丢弃。一项研究现在描述了冷冻电镜结构的停滞复合物准备拆卸,确定方面的异常复杂的识别和剪接质量控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
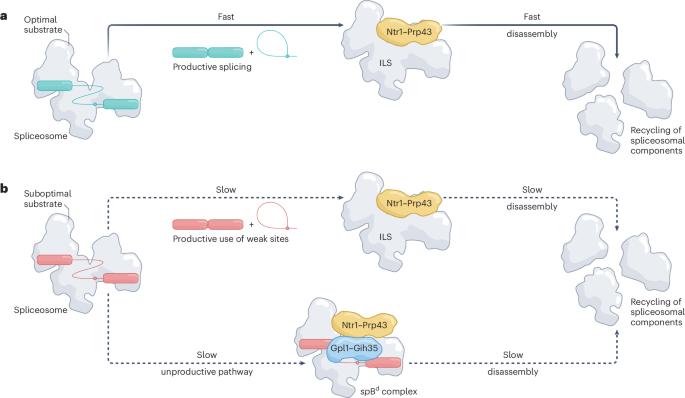

Finding and recycling stalled spliceosomes
Splicing of pre-mRNA requires both high fidelity and enormous sequence flexibility, inevitably resulting in aberrant, stalled complexes that must be discarded. A study now describes cryo-EM structures of stalled complexes poised for disassembly, determining aspects of aberrant complex recognition and splicing quality control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


