镍催化烯系肟酯与二硫代磺酸的对映选择性还原亚氨基二磺化反应
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
提出了一种新的镍催化烯系肟酯与二硫代磺酸的对映选择性还原亚氨基二磺化反应。该反应具有特殊的区域选择性和对映体选择性,具有广泛的底物相容性。手性二硫化吡咯的克级合成和多用途的后续转化突出了这种支架的合成效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
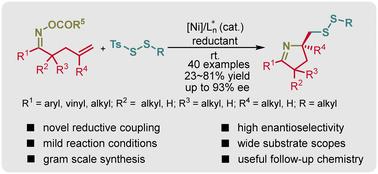
Nickel-catalyzed enantioselective reductive iminodisulfuration of alkene-tethered oxime esters with dithiosulfonate†
A novel nickel-catalyzed enantioselective reductive iminodisulfuration of alkene-tethered oxime esters with dithiosulfonate is presented in this study. This reaction exhibits exceptional regio- and enantioselectivity, showing broad substrate compatibility. Gram-scale synthesis of chiral pyrroline disulfide and versatile subsequent conversions highlight the synthetic utility of this scaffold.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


