白曲霉NRRL 5214的新天然产物及其被色褐链霉菌ATCC 49982糖基化。
IF 2.9
4区 生物学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
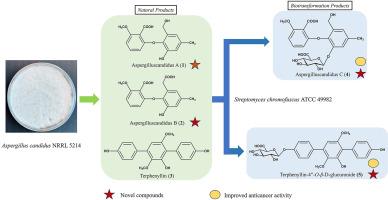
真菌是生物活性天然产物的丰富来源。在这项研究中,我们从真菌菌株Aspergillus candius NRRL 5214中分离和鉴定了两种新的二苯基醚,命名为aspergilluscandius A(1)和aspergilluscandius B(2),以及已知的化合物terphenyllin(3)。化合物1-3的化学结构通过广泛的一维和二维NMR分析进行了表征。化合物1和3随后被放线菌Streptomyces chromofuscus ATCC 49982生物转化为两种新的糖苷,即曲霉C(4)和terphenyllin-4″-O-β-d-葡糖苷(5)。细胞毒性实验表明,糖基化产物4和5对胶质母细胞瘤33细胞株的抑制活性显著提高,IC50分别从8.15±1.09 μM(1)降至5.41±0.30 μM(4),从88.29±10.54 μM(3)降至31.25±4.20 μM(5)。本研究强调假丝酵母NRRL 5214是一个很有前景的天然新产物来源,并提出了利用S. chromofuscus ATCC 49982修饰二苯醚和对三苯化合物以增强其细胞毒性活性的有效策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
New natural products from Aspergillus candidus NRRL 5214 and their glycosylation by Streptomyces chromofuscus ATCC 49982
Fungi represent a rich source of bioactive natural products. In this study, we present the isolation and identification of two new diphenyl ethers, named aspergilluscandidus A (1) and aspergilluscandidus B (2), along with a known compound terphenyllin (3), from the fungal strain Aspergillus candidus NRRL 5214. The chemical structures of compounds 1–3 were characterized through extensive 1D and 2D NMR analysis. Compounds 1 and 3 were subsequently biotransformed into two new glycosides, namely aspergilluscandidus C (4) and terphenyllin-4″-O-β-d-glucuronide (5) by the actinomycete strain Streptomyces chromofuscus ATCC 49982. The cytotoxicity assay revealed that the glycosylated products 4 and 5 exhibited significantly improved activity against the glioblastoma 33 cell line compared to their respective substrates, decreasing the IC50 from 8.15 ± 1.09 μM (1) to 5.41 ± 0.30 μM (4) and from 88.29 ± 10.54 μM (3) to 31.25 ± 4.20 μM (5), respectively. Our study emphasizes A. candidus NRRL 5214 as a promising source of new natural products and presents an effective strategy for modifying both diphenyl ether and p-terphenyl compounds using S. chromofuscus ATCC 49982 to enhance their cytotoxicity activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of bioscience and bioengineering
生物-生物工程与应用微生物
CiteScore
5.90
自引率
3.60%
发文量
144
审稿时长
51 days
期刊介绍:
The Journal of Bioscience and Bioengineering is a research journal publishing original full-length research papers, reviews, and Letters to the Editor. The Journal is devoted to the advancement and dissemination of knowledge concerning fermentation technology, biochemical engineering, food technology and microbiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


