罗伊氏芽孢杆菌细胞表面蛋白肋结构域的化学位移分配。
IF 0.6
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
摘要
Rib结构域是在革兰氏阳性细菌细胞表面蛋白中发现的一种保守结构元件,在细菌毒力中起作用,是疫苗开发的潜在靶点。尽管有高分辨率晶体结构的可用性,但Rib结构域的精确功能作用仍然难以捉摸。在这里,我们报道了罗伊氏乳酸杆菌细胞表面蛋白的Rib结构域的化学位移分配,为理解其在宿主-细菌相互作用中的潜在参与提供了基础步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
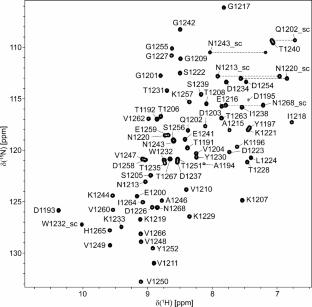
Chemical shift assignments of the rib domain in a cell surface protein from Limosilactobacillus reuteri
The Rib domain, a conserved structural element found in Gram-positive bacterial cell surface proteins, plays a role in bacterial virulence and is a potential target for vaccine development. Despite the availability of high-resolution crystallographic structures, the precise functional role of the Rib domain remains elusive. Here, we report the chemical shift assignments of the Rib domain from a cell surface protein of Limosilactobacillus reuteri, providing a foundational step toward understanding its potential involvement in host-bacteria interactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


