结合生物合成途径和转录因子工程的重建穿梭系统增强l-半胱氨酸产量†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
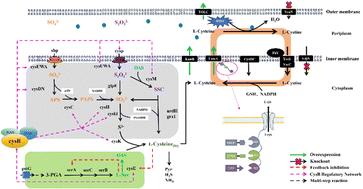
l -半胱氨酸是一种重要的含硫氨基酸,广泛应用于制药、食品、化妆品和饲料工业;它在地球上的硫循环中也起着至关重要的作用。在这里,我们报道了一种无质粒工程大肠杆菌菌株的发展,以提高l -半胱氨酸的产量。最初,l -半胱氨酸/ l -胱氨酸穿梭系统被重组以适应新的工程菌株。对于关键代谢节点CysE,采用基因组多拷贝策略广泛增强其表达,从而增加碳模块的代谢通量。然后构建了l -半胱氨酸的底物通道,以提高生物合成效率。设计了多模块共定位策略,将模块酶与外排系统耦合,协调产物的生物合成和运输。此外,筛选了一个促进硫同化的CysB突变体。通过加强碳硫模块,菌株GCB2在5l生物反应器中产生35.54 g L−1 L-半胱氨酸,葡萄糖产率为0.125 g g−1,硫同化率为92.44%,产率为0.555 g L−1 h−1。据我们所知,这是知名度最高的生产,为未来的工业应用奠定了基础。我们在这项研究中制定的策略也可以应用于其他化学品的生产。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Coupling a rebuild shuttle system with biosynthetic pathway and transcription factor engineering for enhanced l-cysteine production†
l-Cysteine, a vital sulfur-containing amino acid, is extensively utilized in the pharmaceutical, food, cosmetics, and feed industries; it also plays a crucial role in the sulfur cycle on the Earth. Here, we report the development of a plasmid-free engineered Escherichia coli strain for enhanced l-cysteine production. Initially, the l-cysteine/l-cystine shuttle system was restructured to adapt to the new engineered strain. For the critical metabolic node CysE, a genomic multi-copy strategy was employed to enhance its expression extensively, thereby increasing the metabolic flux of the carbon module. A substrate channel for l-cysteine was then constructed to enhance biosynthetic efficiency. The multi-module co-localization strategy was designed to couple the module enzyme with the efflux system, which coordinates the biosynthesis and transport of the product. Moreover, a CysB mutant was screened to promote sulfur assimilation globally. By enhancing the carbon and sulfur module, the strain GCB2 produced 35.54 g L−1 of l-cysteine in a 5 L bioreactor, with a glucose yield of 0.125 g g−1, a sulfur assimilation of 92.44%, and a productivity of 0.555 g L−1 h−1. To our knowledge, this is the highest-known production, laying the foundation for future industrial applications. The strategy we developed in this study can also be applied for the production of other chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


