用草酸亚锡原位生成无枝晶锌阳极的锡保护层
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
水锌离子电池(azib)由于其成本效益、高容量和环境友好性而成为储能设备的热门。然而,锌阳极枝晶生长不受控制和腐蚀严重阻碍了azib的进一步发展。构建保护界面层是缓解和抑制上述问题的一种很有前途的策略。在此,我们提出在锌阳极上涂覆草酸亚锡悬浮液来原位构建锡保护层,从而调节锌的成核位置,诱导Zn(002)晶体平面的沉积,抑制锌枝晶的不可控生长。疏水锡金属保护层隔离了活性水与锌阳极的接触,抑制了锌阳极的腐蚀。结果表明,改进后的Zn//Zn对称电池在1ma cm−2下的循环寿命为2300 h,极化过电位显著降低。用改性Zn阳极组装的MnO2//Zn电池也表现出良好的性能,循环1000次后的比容量为94.74 mA h g−1。这项工作证明了金属盐在开发稳定金属电极方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
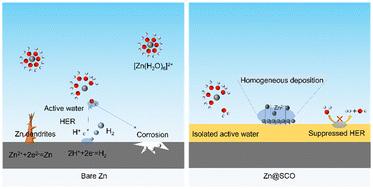
In situ generated tin protective layers from stannous oxalate for dendrite-free zinc anodes†
Aqueous zinc-ion batteries (AZIBs) have become popular for energy storage devices due to their cost-effectiveness, high capacity and environmental friendliness. However, the uncontrolled growth of dendrites and corrosion at the zinc anode have seriously hindered AZIBs’ further development. The construction of protective interface layers is a promising strategy to mitigate and suppress the above problems. Herein, we propose the in situ construction of a protective layer of Sn at the zinc anode by coating a suspension of stannous oxalate, which regulates the zinc nucleation sites and induces the deposition of Zn (002) crystal planes, inhibiting the uncontrolled growth of zinc dendrites. The hydrophobic Sn metal protective layer isolated the contact between the active water and the zinc anode and suppressed the corrosion of the zinc anode. As a result, the modified Zn//Zn symmetric cell has a cycle life of 2300 h at 1 mA cm−2, with a significant reduction in the polarisation overpotential. The MnO2//Zn full cell assembled with the modified Zn anode also showed good performance with a specific capacity of 94.74 mA h g−1 after 1000 cycles. This work exemplifies the potential of metal salts in the development of stable metal electrodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


