生物质明胶衍生的介孔掺氮碳网络及其双功能性,可有效催化可充电锌-空气电池中的氧还原和进化反应
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
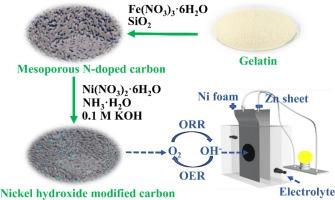
开发高性价比的氧还原反应(ORR)和析氧反应(OER)双功能催化剂是实现可充电锌空气电池商业化的关键。本文利用生物质明胶作为碳和氮的共同来源来制造铁和氮共掺杂的ORR碳催化剂。以二氧化硅为孔模板制备具有大BET比表面积和分层结构的介孔碳网络。此外,在氨的缓冲作用下,氢氧化镍颗粒以高分散性锚定在碳网络上,使碳材料具有OER活性。合成的FeGeSi-A2-Ni催化剂在5 mA cm−2下ORR半波电位为0.8693 V, OER过电位为398 mV,具有良好的ORR和OER双功能。实际应用中,由FeGeSi-A2-Ni组装的可充电锌-空气电池表现出高度稳定的可充电性,放电电压为~ 1.15 V,充电电压为~ 2.05 V,明显优于Pt/C-RuO2电池。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biomass gelatin-derived mesoporous nitrogen-doped carbon networks and its bifunctionality to effectively catalyze oxygen reduction and evolution reactions for rechargeable zinc-air battery
Developing cost-effective bifunctional catalysts with high performance to oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is key to the commercialization of rechargeable zinc-air batteries. Herein, biomass-derived gelatin is utilized as the common source of carbon and nitrogen to fabricate an iron and nitrogen co-doped carbon catalyst to ORR. Mesoporous carbon networks are generated by using silicon dioxide as the pore template in the preparation of the catalyst with a large BET surface area and hierarchical structure. Furthermore, nickel hydroxide particles are anchored on the carbon networks with high dispersion under the buffering of ammonia, which endows the carbon material with OER activity. The resultant catalyst FeGeSi-A2-Ni provides satisfactory bifunctionality to ORR and OER with 0.8693 V of ORR halfwave potential and 398 mV of OER overpotential at 5 mA cm−2. Practically, the rechargeable zinc-air battery assembled from the FeGeSi-A2-Ni exhibits highly stable rechargeability with ∼ 1.15 V of discharge voltage and ∼ 2.05 V of charge voltage in the total process, being obviously excellent than the battery of Pt/C-RuO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


