利用功能化咪唑基离子液体从模拟油中分离吡啶的有效萃取和理论见解
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
吡啶是一种有价值的化学物质,从经济和环境的角度来看,从煤焦油中回收和从化石燃料中去除吡啶是必不可少的。本研究首次采用具有腈和醚功能化咪唑基阳离子的离子液体(ILs),与硫氰酸盐(SCN−)和硫酸氢(HSO4−)阴离子配对,用于模拟油的有效脱氮。用脱氮效率(RE)评价了脱氮性能。综合比较,两种IL在模拟燃油中具有良好的正己烷萃取效果,只有阴离子为HSO4−的IL在模拟煤焦油中具有良好的正己烷和甲苯萃取效果。在最佳脱氮条件下,以HSO4−为阴离子的IL的RE大于94%:IL与模拟油的质量比(MIL: m模拟油)为1:4;提取时间,15 min;萃取温度为298 K,初始吡啶浓度为5wt %。在相同条件下,以SCN−为阴离子的IL的RE大于75%。此外,利用量子化学计算方法研究了分子静电势、相互作用能和密度梯度。这些结果通过评价它们之间的有利相互作用来分析il对吡啶具有较高的亲和力,从而证实了实验结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
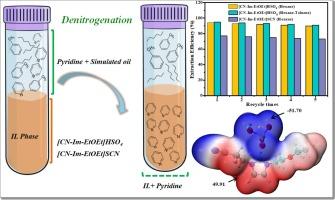
Efficient extraction and theoretical insights into the separation of pyridine from simulated oil using functionalized imidazolium-based ionic liquids
Pyridine is a valuable chemical and its recovery from coal tar and removal from fossil fuels are essential from economic and environmental perspectives. For the first time, this study employed ionic liquids (ILs) featuring nitrile and ether-functionalized imidazolium-based cations, paired with thiocyanate (SCN−) and hydrogen sulfate (HSO4−) anions, for the effective denitrogenation of simulated oil. The denitrogenation performance was evaluated using removal efficiency (RE). A comprehensive comparison of both ILs demonstrated excellent extraction efficiency in the simulated fuel oil with hexane, and only the IL with the anion of HSO4− exhibited good extraction performance in the simulated coal tar oil with hexane and toluene. The RE of the IL with the anion of HSO4− exceeded 94 %, under optimal denitrogenation conditions: mass ratio of IL to model oil (MIL:MSimulated oil), 1:4; extraction time, 15 min; extraction temperature, 298 K and initial pyridine concentration of 5 wt%. The RE of the IL with SCN− as an anion was over 75 % under similar conditions. Additionally, the molecular electrostatic potentials, interaction energies, and reduced density gradient were investigated for the ILs and pyridine using quantum chemical calculations. These results confirmed the experimental findings by analyzing the comparatively higher affinity of ILs for pyridine through the evaluation of favorable interaction between them.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


