可控中温合成高熵PtIrCoNixFe1-x的优越析氢反应
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
铂(Pt)因其在析氢反应(HER)中的显著催化活性而闻名;然而,其巨大的成本限制了其广泛使用。Pt与过渡金属的结合优化了其电子结构,提高了催化效率,同时减少了对Pt的依赖。本研究报告了通过中温溶剂热法合成PtIrCoNixFe1-x高熵合金纳米颗粒(HEA NPs),为在降低Pt含量的情况下获得高HER活性提供了一种经济高效的解决方案。结构分析(PXRD, XPS, TEM, EDX制图)证实形成了元素分布均匀的单相HEAs。通过调整Ni和Fe的比例,PtIrCoNi2·9Fe3.9 HEA NPs样品表现出优异的HER活性,在碱性介质中表现出27 mV的低过电位和37.8 mV dec−1的Tafel斜率,优于商用Pt/C。DFT模拟表明HEAs的HER活性增强是由于d-d电子相互作用和多个活性位点降低了能量势垒。Ir-5d轨道促进电子转移,而Pt-5d轨道充当电子储存库。靠近费米能级的Ni-3d、Fe-3d和Co-3d轨道形成了一个电子耗尽中心,进一步提高了HER的活性。这些发现突出了PtIrCoNixFe1-x HEA NPs作为可调HER电催化剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
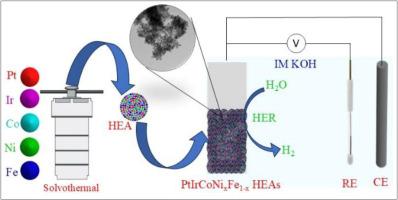
Controllable moderate-temperature synthesis of high entropy PtIrCoNixFe1-x for superior hydrogen evolution reaction
Platinum (Pt) is renowned for its remarkable catalytic activity in the hydrogen evolution reaction (HER); however, its significant cost constrains its widespread use. Integrating Pt with transition metals optimizes its electronic structure, enhancing catalytic efficiency while reducing Pt reliance. This study reports the synthesis of PtIrCoNixFe1-x high-entropy alloy nanoparticles (HEA NPs) via a moderate-temperature solvothermal method, offering a cost-efficient solution for achieving high HER activity with reduced Pt content. Structural analyses (PXRD, XPS, TEM, EDX mapping) confirm the formation of single-phase HEAs with evenly distributed elements. By adjusting Ni and Fe ratios, PtIrCoNi2·9Fe3.9 HEA NPs sample show superior HER activity, outperforming commercial Pt/C with a low overpotential of 27 mV at −10 mA cm−2 and a Tafel slope of 37.8 mV dec−1 in alkaline media. DFT simulations reveal that the enhanced HER activity of the HEAs is due to d-d electron interactions and multiple active sites that lower energy barriers. Ir-5d orbitals facilitate electron transfer, while Pt-5d orbitals act as an electron reservoir. Ni-3d, Fe-3d, and Co-3d orbitals near the Fermi level create an electron depletion center, further improving HER activity. These findings highlight the potential of PtIrCoNixFe1-x HEA NPs as tunable HER electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


