用分子模型催化剂鉴定高自旋羟基配位Fe3+N4为酸性氧还原活性中心
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Fe-N-C催化剂是酸性氧还原反应(ORR)中最有希望替代Pt的催化剂,但其活性中心的电子结构仍然难以捉摸。本文合成并表征了一种共轭桥联酞菁铁(FePc)二聚体模型催化剂,该催化剂具有相同的铁位和与实际催化剂相当的催化活性。具有轴向羟基配体的高自旋三价FeN4为活性态,记为OH-Fe3 +N4 (S = 5/2)。而单体和非共轭二聚体表现为OH-Fe3 +N4 (S = 3/2)态,ORR中间体吸附能过高。聚合FePc由35%的S = 5/2态和65%的Fe2+N4 (S = 0或1)态组成,表现出普遍较弱的吸附能。过强和过弱的吸附能都会阻碍ORR。理论计算表明,铁与共轭碳平面之间的π-d相互作用决定了自旋态。本研究将有助于铁基ORR催化剂的精确设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
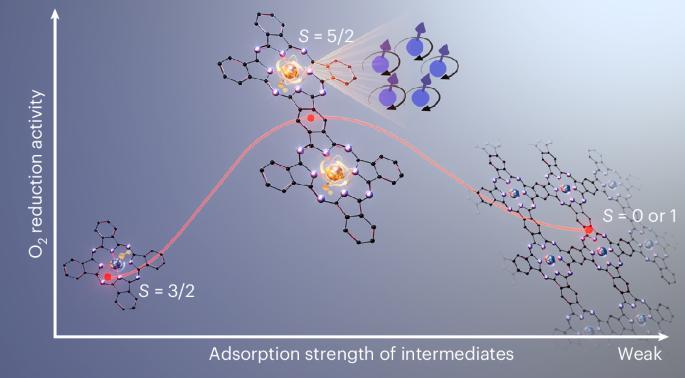

Identifying high-spin hydroxyl-coordinated Fe3+N4 as the active centre for acidic oxygen reduction using molecular model catalysts
Fe–N–C catalysts are the most promising alternative to Pt for the acidic oxygen reduction reaction (ORR), yet the electronic structure of their active centres remains elusive. Here we synthesize and characterize a conjugate-bridged iron phthalocyanine (FePc) dimer model catalyst with identical Fe sites and catalytic activity comparable to actual catalysts. A high-spin trivalent FeN4 with an axial hydroxyl ligand, denoted as OH–Fe3+N4 (S = 5/2), is identified as the active state. By contrast, monomer and non-conjugated dimer manifest the OH–Fe3+N4 (S = 3/2) state with an excessive adsorption energy of ORR intermediates. Polymerized FePc is composed of 35% of the S = 5/2 state and 65% of the Fe2+N4 (S = 0 or 1) state, showing a general weaker adsorption energy. Both overly strong and weak adsorption energy hinder the ORR. Theoretical calculations indicate that π–d interaction between Fe and the conjugated carbon plane dictates the spin state. This study will help to precisely design Fe-based ORR catalysts. Single-atom Fe–N–C catalysts are the most promising alternative to Pt-group metal catalysts for the cathodic oxygen reduction reaction in fuel cells, but while the chemical environment of their active centres is well understood, their electronic structure remains elusive. Now, using molecular model catalysts, a high-spin trivalent FeN4 site with an axial hydroxyl ligand is identified as the active site in Fe–N–C catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


