pom插层NiFe-LDH作为增强型OER催化剂在安培电流密度下高效和持久的水电解
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
高效、经济的电催化剂对于通过电化学水分解大规模制氢至关重要,特别是在工业规模的电流密度下。本研究采用一步水热法原位合成了由keggin型多金属氧酸盐(POM) H3PW12O40 (PTA)插入镍铁层状双氢氧化物(NiFe-LDH-PTA)的非均相电催化剂。物理化学表征和理论计算证实,将PTA插入到NiFe-LDH层中会触发LDH主体和PTA客体之间的电子相互作用,调节Ni和Fe活性位点的电子结构,减轻活性物质的溶解。利用这些主客体相互作用,即使在工业规模条件下,nfe - ldh - pta也显示出卓越的析氧反应(OER)催化活性。值得注意的是,催化剂只需要342±9 mV的过电位就可以达到1000 mA cm-2的电流密度,并在该电流密度下保持500小时的稳定运行。此外,组装的阴离子交换膜(AEM)电解槽在60°C的低电池电压1.93 V下实现了1000 mA cm-2的工业规模电流密度。这项工作为合理设计适合工业应用的高性能ldh基OER电催化剂提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
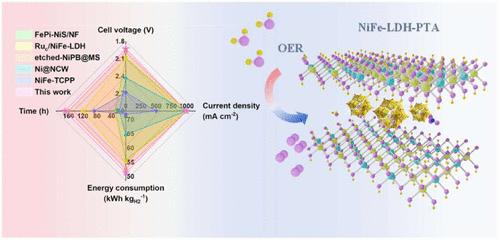
POM-Intercalated NiFe-LDH as Enhanced OER Catalyst for Highly Efficient and Durable Water Electrolysis at Ampere-Scale Current Densities
Efficient and cost-effective electrocatalysts are essential for large-scale hydrogen production via electrochemical water splitting, particularly at industrial-scale current densities. In this study, a heterogeneous electrocatalyst, comprising Keggin-type polyoxometalate (POM), H3PW12O40 (PTA), intercalated into nickel–iron layered double hydroxides (NiFe-LDH-PTA) was synthesized in situ using a one-step hydrothermal method. The intercalation of PTA into the NiFe-LDH layers triggers electronic interactions between the LDH host and PTA guest, modulating the electronic structure of the Ni and Fe active sites and mitigating the dissolution of active species, as confirmed by physicochemical characterizations and theoretical calculations. Leveraging these host–guest interactions, NiFe-LDH-PTA demonstrates exceptional catalytic activity for the oxygen evolution reaction (OER), even under industrial-scale conditions. Notably, the catalyst requires an overpotential of only 342 ± 9 mV to achieve a current density of 1000 mA cm–2 and maintains stable operation at this current density for 500 h. Furthermore, the assembled anion-exchange membrane (AEM) electrolyzer achieves an industrial-scale current density of 1000 mA cm–2 at a low cell voltage of 1.93 V at 60 °C. This work provides valuable insights into the rational design of high-performance LDH-based OER electrocatalysts tailored for industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


