预湿方解石中的除油:用傅里叶变换红外和x射线光电子能谱研究活性与非活性离子
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在盐下油藏中,天然岩石与地层水(FW)和矿物油同时相互作用。采用傅里叶变换红外光谱和x射线光电子能谱技术,研究具有FW和去矿化水(DW)的预水合方解石在其表面的油吸附(去除)作用是合适的模型体系。对直接受低盐度(LS)水、FW和DW影响的新鲜解理方解石的化学成分的初步表征表明,方解石在与DW和稀释的LS水(LS100)接触后发生了(1)表面溶解,其CO32 -基团的振动带分裂(1);(2)方解石e通过Mg的掺入、表面水锚定和方解石/FW界面的盐沉积形成而发生了部分化学修饰。Nujol对原始方解石和(FW, DW)预湿方解石进行了预处理,发现Nujol在FW预水表面的CH2和CH3振动带强度总体大于方解石/DW/油和方解石/油界面。方解石/油、方解石/FW/油和方解石/DW/油与LS水的最终调理结果表明,在离子强度为0.2 mol/L的盐水溶液中,与预水化无关,方解石/油的除油效果更好。我们的研究结果表明,方解石化学成分或溶解的改变会影响随后的油吸附和去除,因此,在使用LS水提高采收率的过程中,盐水离子强度与油和表面成分之间存在竞争作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
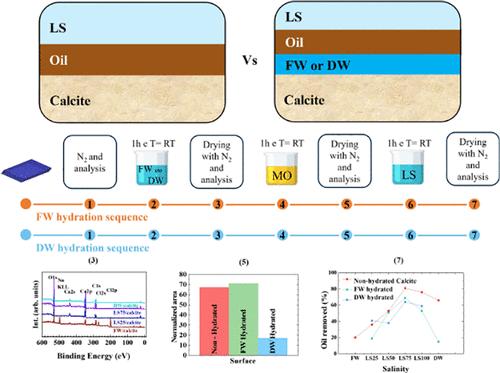
Oil Removal in Prewet Calcite: Active Versus Inactive Ions Investigated by a Fourier Transform Infrared and X-ray Photoelectron Spectroscopy Study
In presalt reservoirs, natural rocks interact simultaneously with formation water (FW) and mineral oil. The prehydrated calcites with FW and demineralized water (DW) are suitable model systems to investigate oil adsorption (removal) on (from) their surfaces by Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy. Preliminary characterization of the chemical composition of fresh cleaved calcite conditioned directly with low salinity (LS) waters, FW, and DW indicates that calcite undergoes (i) surface dissolution once in contact with DW and diluted LS water (LS100) as testified by the split of ν3 vibration bands of the CO32– group and (ii) partial chemical modification of calcite e through Mg incorporation, water anchoring at the surface, and salt deposit formation at the calcite/FW interface. Pristine and (FW, DW) prewet calcites were conditioned with Nujol, and an overall larger CH2 and CH3 vibration band intensity of Nujol was observed on the FW prehydrated surface than calcite/DW/oil and calcite/oil interfaces. The final conditioning of calcite/oil, calcite/FW/oil, and calcite/DW/oil with LS water ended up with greater oil removal for a saline solution of 0.2 mol/L ion strength, independent of the prehydration. Our results indicate that altered calcite chemical composition or dissolution affects subsequent oil adsorption and removal, and, therefore, there is a competitive role between brine ion strength and oil and surface compositions in the enhanced oil recovery process using LS water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


