细胞内合成纳米硒三重功能益生菌通过调节巨噬细胞表型和调节肠道微生物群治疗结肠炎
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
巨噬细胞表型失调作为结肠炎的主要病因,不仅增强了氧化应激,加剧了炎症反应,而且与肠道微生物菌群失调密切相关。要想有效治疗结肠炎,需要同时解决这三个问题,但这并不令人满意。在此,我们开发出了治疗结肠炎的 "三鸟合一 "益生菌,命名为 Se@EcN-C2/A2。大肠杆菌 Nissle 1917(EcN)是一种临床认可的益生菌,它通过生物矿化作用在细胞内合成硒(Se)纳米颗粒,从而得到 Se@EcN。在 Se@EcN (Se@EcN-C2/A2)表面包覆乙二醇壳聚糖和海藻酸钠,使益生菌对恶劣的胃肠道环境有很强的抵抗力,并对结肠发炎部位有很强的粘附和靶向能力,便于 M1 巨噬细胞吸收。Se@EcN-C2/A2被代谢为SeCys2和MetSeCys,参与GPX2和TXNRD1的合成,从而清除反应氧物种,抑制Toll样受体和核因子κB信号通路,抑制炎症反应,并通过激活PI3K/AKT信号通路将M1巨噬细胞极化为M2表型。在DSS诱导的结肠炎小鼠中,Se@EcN-C2/A2发挥了令人满意的治疗和预防效果,包括清除氧化应激和调节巨噬细胞表型,以抑制炎症反应和恢复肠道屏障功能。此外,结肠中的活益生菌EcN通过降低志贺氏菌的丰度和增加乳酸杆菌和双歧杆菌的丰度,有效地调节了微生物菌群失调。本文章由计算机程序翻译,如有差异,请以英文原文为准。
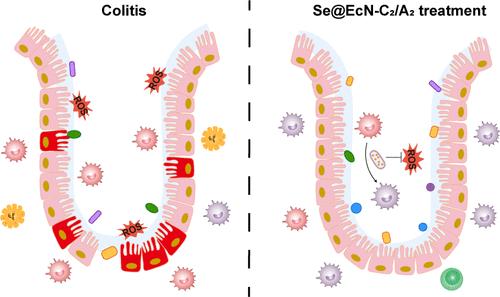
Triple-Functional Probiotics with Intracellularly Synthesized Selenium Nanoparticles for Colitis Therapy by Regulating the Macrophage Phenotype and Modulating Gut Microbiota
The dysregulated macrophage phenotype, as the main cause of colitis, not only enhanced oxidative stress to exacerbate inflammatory responses but was closely related with gut microbial dysbiosis. It was needed to simultaneously address the three issues for the effective treatment of colitis, but it was not satisfied. Here, we developed “three-birds-one-stone” probiotics, named Se@EcN-C2/A2, for colitis treatment. Escherichia coli Nissle 1917 (EcN), a clinically approved probiotic, was used to intracellularly synthesize selenium (Se) nanoparticles by biomineralization, giving Se@EcN. Coating glycol chitosan and sodium alginate on the surface of Se@EcN (Se@EcN-C2/A2) endowed probiotics with high resistance to the harsh gastrointestinal tract environment and strong adhesion and targeting ability to the inflamed site of the colon to facilitate the uptake by M1 macrophages. Se@EcN-C2/A2 was metabolized to SeCys2 and MetSeCys to be involved in the synthesis of GPX2 and TXNRD1, which led to reaction oxygen species clearance to inhibit Toll-like receptor and nuclear factor κB signaling pathways to suppress inflammatory response and polarize M1 macrophages to M2 phenotypes by activating PI3K/AKT signaling pathways. In DSS-induced colitis mice, Se@EcN-C2/A2 exerted satisfactory therapeutic and prophylactic effects, including scavenging oxidative stress and regulating macrophage phenotypes to suppress inflammatory response and restore gut barrier functions. Moreover, the living probiotic EcN in the colon effectively regulated microbial dysbiosis by decreasing the abundance of Escherichia-Shigella and increasing the abundance of Lactobacillus and Bifidobacterium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


