TRPM7介导的Mg2+内流触发肌肉干细胞激活的启动
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
肌肉卫星细胞(musc)对骨骼肌损伤的环境信号立即作出反应。尽管对肌肉再生进行了数十年的研究,但触发musc从静止状态向活跃状态转变的特定分子因子在很大程度上仍未被确定。在这里,我们发现瞬时受体电位美拉抑素7 (TRPM7),一个Mg2+渗透性离子通道,作为MuSC激活的关键调节因子。MuSC中的Trpm7缺失减少了Mg2+内流,损害了肌纤维再生,导致MuSC数量减少和再生过程中的细胞周期停滞。这些变化与mTOR信号中断有关,mTOR信号中断驱动musc从G0阶段过渡到GAlert阶段。此外,trpm7缺失的musc表现出受损的早期反应,包括静止的投射收缩和AP-1诱导。Mg2+的补充修复了这些缺陷,恢复了正常的MuSC激活。我们的研究结果揭示了一个以前未被认识的机制,其中Mg2+通过TRPM7的渗透对于MuSC激活和有效的骨骼肌再生至关重要,强调了TRPM7是肌肉修复的关键调节剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
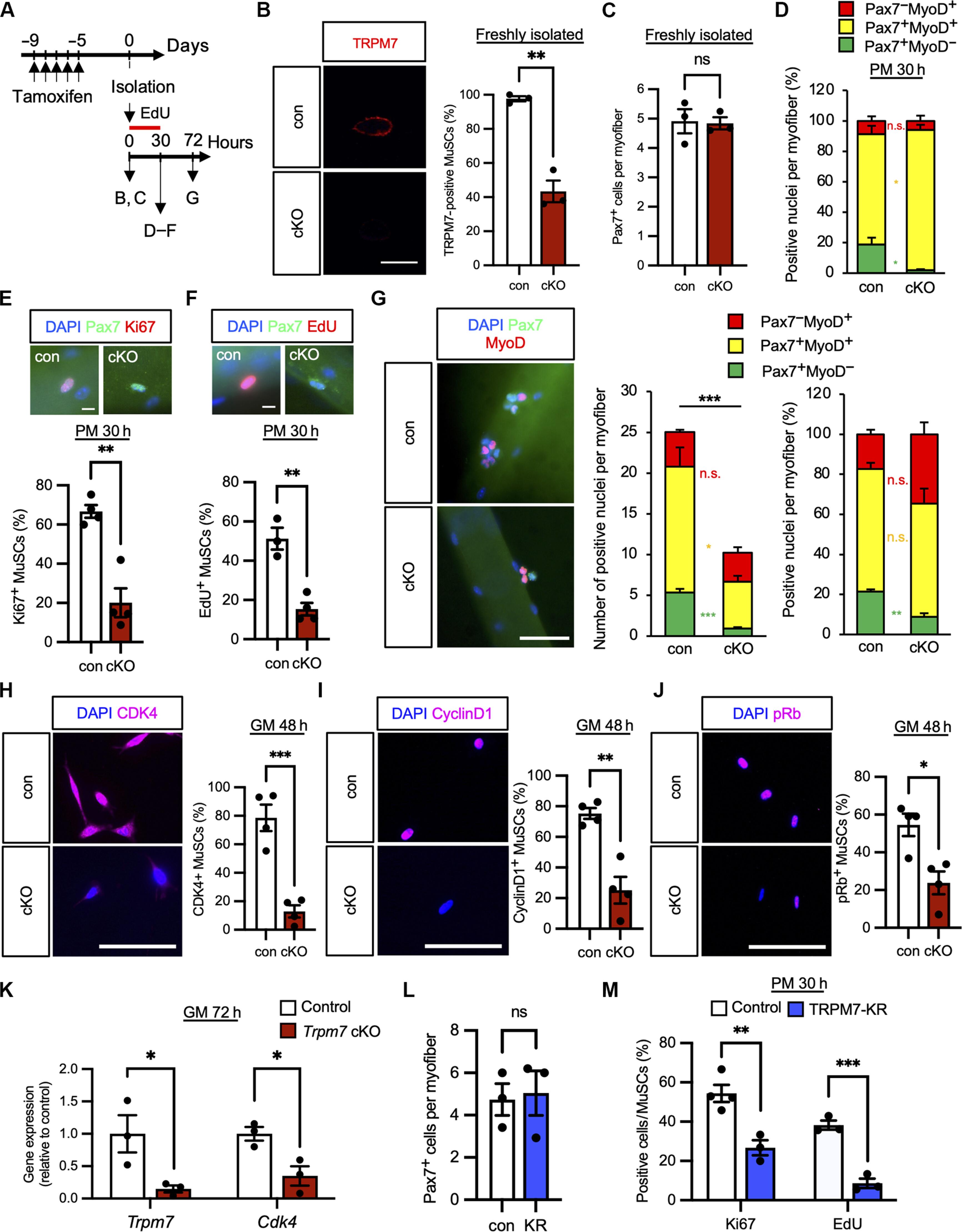
Mg2+ influx mediated by TRPM7 triggers the initiation of muscle stem cell activation
Muscle satellite cells (MuSCs) respond immediately to environmental cues upon skeletal muscle injuries. Despite decades of research into muscle regeneration, the specific molecular factors that trigger the transition of MuSCs from a quiescent to an active state remain largely unidentified. Here, we identify transient receptor potential melastatin 7 (TRPM7), an Mg2+-permeable ion channel, as a critical regulator of MuSC activation. Trpm7 deletion in MuSCs reduced Mg2+ influx, impairing myofiber regeneration and leading to decreased MuSC numbers and cell cycle arrest during regeneration. These changes were linked to disrupted mTOR signaling, which drives the transition of MuSCs from G0 to GAlert phase. In addition, Trpm7-deficient MuSCs exhibited impaired early responses, including quiescent projection retraction and AP-1 induction. Mg2+ supplementation rescued these defects, restoring normal MuSC activation. Our findings reveal a previously unrecognized mechanism where Mg2+ permeation through TRPM7 is essential for MuSC activation and efficient skeletal muscle regeneration, highlighting TRPM7 as a critical regulator of muscle repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


