波长解析催化周转频率和确定替代质子供体在太阳能燃料形成反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
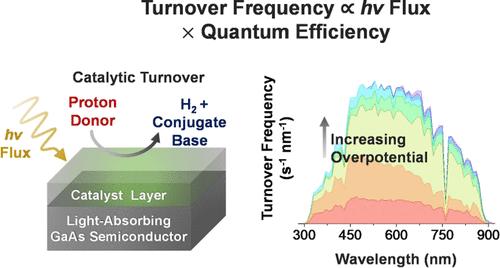
在电催化中,催化剂通过降低达到预期反应速率的过电位要求,将驱动电极表面氧化还原半反应所需的能量降至最低。出现了两个比较分子电催化剂性能的基准参数。它们是最大周转频率──相当于化学催化的速率常数kcat──和有效过电位──激活电极表面一半催化剂所需的电位。在这里,我们将这些概念和相关的分析技术扩展到光电合成反应,其中除了电子和化学底物外,还需要光子作为试剂。我们的研究结果表明,如果观察到的最大反应速率受到光子通量的限制,而不是固有的催化性能,那么与电催化剂基准测试相关的方法如何导致在光活化组件中运行的催化组分的错误度量。这项工作利用了氢演化的模型组件,并强调了应用电化学偏压电位、pH效应和照明强度的复杂性,包括在相对较低的过电位下,缓冲物质如何胜过水作为质子/化学底物的来源。我们还介绍了一种将照明强度与光电合成转换频率联系起来的通用而有用的形式,使我们能够从实验数据中生成我们称之为波长分辨TOF图和光电合成tafel样图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Wavelength-Resolving Catalytic Turnover Frequencies and Identifying Alternate Proton Donors in Solar-Fuel-Forming Reactions
In electrocatalysis, catalysts minimize the energy needed to drive redox half-reactions at electrode surfaces by lowering the overpotential requirement for achieving a desired reaction rate. Two benchmark parameters have emerged for comparing the performance of molecular electrocatalysts. These are the maximum turnover frequency─equivalent to the rate constant for chemical catalysis, kcat─and the effective overpotential─which is the potential required to activate half the population of catalysts at an electrode surface. Herein, we extend these concepts and related analysis techniques to photoelectrosynthetic reactions, where, in addition to electrons and chemical substrates, photons are required as reagents. Our results show how approaches relevant to benchmarking electrocatalysts can result in erroneous metrics for catalytic components operating in light-activated assemblies if the observed maximum reaction rates are limited by fluxes of photons rather than inherent catalytic properties. This work utilizes a model assembly for hydrogen evolution and highlights the complexity of applied electrochemical bias potentials, pH effects, and illumination intensities, including how, at relatively low overpotentials, buffering species can outcompete water as a source of protons/chemical substrates. We also introduce a general yet useful formalism relating illumination intensities to photoelectrosynthetic turnover frequencies, enabling the generation of what we term wavelength-resolved TOF plots and photoelectrosynthetic Tafel-like plots from experimental data.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


