碳-碘键周围稳定且反应迅速的反旋异构
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
旋回异构是一种独特的手性形式,由受限制的键旋转引起,丰富了分子多样性,在药物发现、催化和材料科学中发挥着关键作用。虽然基于第二行元素(例如,双芳基)的手性旋向异构是常见的,但围绕较重元素的长而灵活的轴的稳定旋向异构仍然很少见。在这里,我们引入了稳定的atropisomer,其特征是碳-碘键作为唯一的手性轴,通过将刚性的苯并碘唑支架与大块的熔融芳基配对来实现。这些分子表现出强大的热收缩异构性,旋转势垒超过30 kcal mol-1,外消旋半衰期超过50年。值得注意的是,这些C-I抗缩异构体表现出酸反应性外消旋速率随酸度变化,从而实现半静态或动态抗缩异构。这一特征使得19F NMR能够表征它们与弱手性酸的对映选择性相互作用以及它们在强手性酸介导下的去消酰基化。本文章由计算机程序翻译,如有差异,请以英文原文为准。

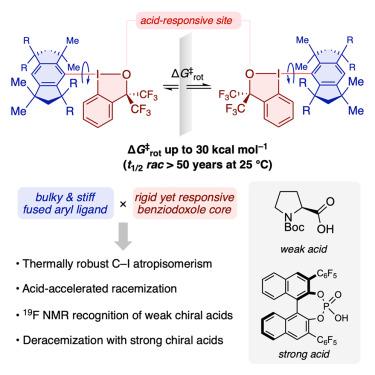
Stable and responsive atropisomerism around a carbon–iodine bond
Atropisomerism, a unique form of chirality arising from restricted bond rotation, enriches molecular diversity and plays a pivotal role in drug discovery, catalysis, and materials science. Although atropisomers with chiral axes based on second-row elements (e.g., biaryls) are common, stable atropisomerism around a long, flexible axis involving heavier elements remains rare. Here, we introduce stable atropisomers featuring a carbon–iodine bond as a sole chiral axis, achieved through pairing a rigid benziodoxole scaffold with a bulky fused aryl group. These molecules exhibit thermally robust atropisomerism, with rotational barriers over 30 kcal mol–1 and racemization half-lives surpassing 50 years. Notably, these C–I atropisomers exhibit acid-responsive racemization rates that vary with acidity, enabling semi-static or dynamic atropisomerism. This feature enables the use of 19F NMR to characterize their enantioselective interactions with weak chiral acids and their deracemization mediated by strong chiral acids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


